New research shows how human cells can be effectively 'recharged' by replacing their internal batteries – microscopic power stations called mitochondria – and the discovery could have wide-ranging benefits across healthcare and medical treatments.
The stacks of mitochondria in most of our cells naturally decline in numbers, slow down, and wear out with age. Once they start operating below peak capacity, they can contribute to multiple diseases everywhere from the heart to the brain.
In this latest study, researchers from Texas A&M University used special flower-shaped particles called nanoflowers to scavenge damaging oxygen molecules, triggering genes that increase the number of mitochondria in human stem cells.
Related: Mitochondria Dump Their Rubbish DNA, And It Could Be Costing Us Our Health
Crucially, those energy-boosted stem cells could then share their mitochondria with old and damaged neighboring cells. It's more of a battery swap than a recharge, but it means existing cells that have stopped functioning can get back to work.
"We have trained healthy cells to share their spare batteries with weaker ones," says biomedical engineer Akhilesh Gaharwar.
"By increasing the number of mitochondria inside donor cells, we can help aging or damaged cells regain their vitality – without any genetic modification or drugs."
In the video below, recipient cells (green) receive new mitochondria (red) from healthy stem cells. (Courtesy of Dr. Akhilesh K. Gaharwar)
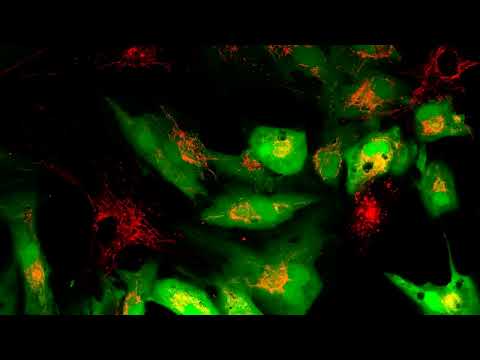
Made from the compound molybdenum disulfide, the nanoflowers were developed with tiny holes that made them act like sponges capable of soaking up stressful reactive oxygen species in target tissues. This removal was found to trigger the expression of genes that kick mitochondria production up several notches in the experiment's stem cells.
Stem cells are naturally built to share mitochondria, but in these lab experiments, they had many more power stations to spare than normal, which improved the recharging effect on other cells.
Around two times more mitochondria were shared than would normally be expected, the researchers report, and smooth muscle cells, found in the heart, increased by three- to four-fold. In heart cells exposed to damaging chemotherapy, the survival rate of the treated cells improved significantly.
The researchers suggest the approach could be used to rejuvenate cells anywhere in the body: close to the heart for cardiovascular problems, for example, or directly into muscle for cases of muscular dystrophy.
"It's pretty promising in terms of being able to be used for a whole wide variety of cases, and this is just kind of the start," says geneticist John Soukar.
"We could work on this forever and find new things and new disease treatments every day."
This is all very positive, but the researchers themselves admit they're still at the early stages. While the current study supports the possibility of using nanoparticles to enhance mitochondria transfer, the next step is to get it working in animals and people.
Those future tests should tell us more about where the beneficial stem cells could be implanted in the body, and what level of dose would be safe and appropriate. The longer-term impacts of the process also need to be studied.
"This is an early but exciting step toward recharging aging tissues using their own biological machinery," says Gaharwar.
"If we can safely boost this natural power-sharing system, it could one day help slow or even reverse some effects of cellular aging."
The research has been published in PNAS.

