Nanoscopic proteins made from the antibodies of animals like camels and llamas can penetrate into cellular spaces like no other antibodies can. Mounting evidence suggests these tiny molecules could even be put to work protecting the brain from difficult-to-treat conditions like Alzheimer's and schizophrenia.
In a new paper, a team of scientists from the Centre National de la Recherche Scientifique (CNRS) in France explains where the research has gotten us so far, and what lies ahead.
The CNRS experts believe their small size may make them ideal for reaching into and treating the brain with fewer side-effects, though so far, the four nanobody therapies available have been approved only for treating other parts of the body.
Related: Ozempic Literally Came From a Monster – And It's Not Alone
Antibodies are proteins that our immune system uses to tag unwanted materials in the body – viruses, toxins, and the like – as trash to be removed by our biological cleanup crew.
Nanobodies are stripped-down versions of these proteins, and their sleek, stealthy build means they can squeeze between a virus's defenses to disarm its most dangerous parts.
Members of the camelid family (including llamas, camels, and alpacas) naturally produce smaller antibodies than humans. Scientists have further refined these in the lab to be about 10 times smaller than the common Y-shaped Immunoglobulin G antibody.
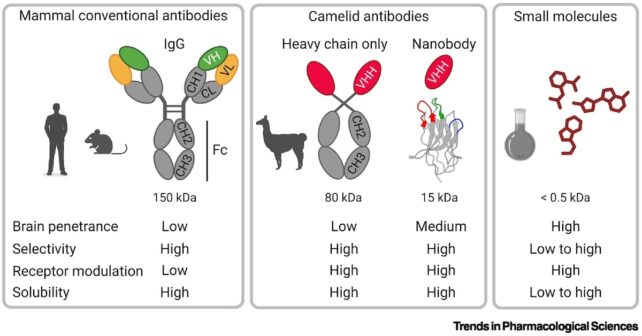
While sharks are also known to produce nanobodies, it's our mammalian relatives whose biological machinery is best positioned to supplement our comparatively clunky immune systems.
Nanobodies made from camelids have already shown promise in protecting humans from influenza A and B, the gastro-causing norovirus, COVID-19, and even HIV.
Until recently, it seemed nanobodies could not be used to treat brain disorders because human kidneys eliminate them from the bloodstream before they even get a chance to reach their target destination. It also seemed they may not readily cross the blood brain barrier: a decisive checkpoint for any drugs targeting the brain.
But newer studies have overcome these challenges, with animal model lab experiments showing that engineered nanobodies can cross the blood brain barrier to target and eliminate key markers of Alzheimer's disease thought to drive symptoms, tau and amyloid beta.
"Camelid nanobodies open a new era of biologic therapies for brain disorders and revolutionize our thinking about therapeutics," says Philippe Rondard, neuropharmacologist at CNRS. "We believe they can form a new class of drugs between conventional antibodies and small molecules."
But before these drugs get anywhere near a living human brain, scientists will need to evaluate their stability, proper folding, and ensure the absence of aggregation, Rondard says.
"These are highly soluble small proteins that can enter the brain passively," says functional genomicist Pierre-André Lafon. "By contrast, small-molecule drugs that are designed to cross the blood-brain barrier are hydrophobic in nature, which limits their bioavailability, increases the risk of off-target binding, and is linked to side effects."
We also need to know more about exactly how they're crossing the blood brain barrier, and how long they hang around in the brain, to ensure proper dosage. Scientists will also need to develop clinical-grade, stable formulations that can survive long-term storage and transport, from lab to patient.
"Our lab has already started to study these different parameters for a few brain-penetrant nanobodies and has recently shown that conditions of treatment are compatible with chronic treatment," Lafon adds.
Perhaps one day we will be thanking camels for saving our precious memories – but it's still a long way off.
This research was published in Trends in Pharmacological Sciences.

