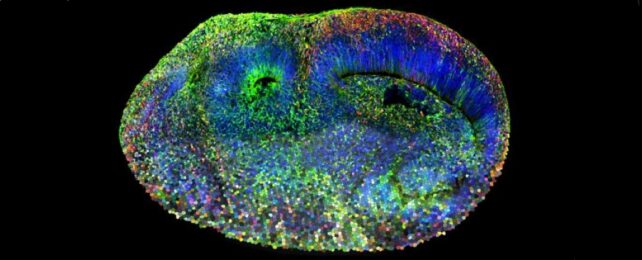
A new scientific technique using brain organoids – mini artificial brains grown in the lab – is revealing the genetic origins of autism spectrum disorder (ASD) hidden within our brains.
Called CHOOSE (which stands for CRISPR–human organoids–single-cell RNA sequencing), the technique combines elaborate genetics and quantitative bioinformatics to study mutations of genes known to be high risk for autism, and how those mutations lead to specific cell changes in the fetal brain.
Because each individual cell in the tiny in vitro brain carries only one mutation (at most) for a specific high-risk gene, the effects of different mutations can be analyzed simultaneously while the cell in question divides and multiples in the growing organoid.
This reveals the consequences of multiple mutations in one experiment, dramatically shortening the time required for analysis, the researchers say.
"With this high-throughput methodology, we can systematically inactivate a list of disease-causing genes. As the organoids carrying these mutations grow, we analyze the effect of each mutation on the development of each cell type," says molecular and cellular pharmacologist Chong Li from the Institute of Molecular Biotechnology (IMBA) in Austria, one of the research groups who developed the technique.
Focusing on 36 known high-risk autism genes, the researchers discovered that while these genes share many common molecular mechanisms, the effect of these mechanisms on different cell types can be distinctly different – making certain cell types vulnerable to autism-related mutations in the fetal brain.
Neural progenitors – founder cells that generate neurons, the nerve cells in our brain – are particularly susceptible to mutations, Li explains, highlighting these cell types as candidates for future study.
The development of our oddly wrinkled brain is an exquisitely complicated process, yet it's this very complexity that gives so many opportunities for brain development to go awry.
Until recently, scientists have relied on autopsy tissues, brain scans, and animal models to understand the brain's inner workings (after all, no one wants their brain tissues poked, prodded, and possibly dissected while they're alive, do they?).
Organoids heralded a new age.
And while blobs of artificial brain might sound Frankenstein's monster-ish, derived from stem cells and growing no bigger than 4 mm (0.15 inches) on a petri dish, they are generally considered models for particular aspects of brain development rather than models of the brain itself.
"Studying the genetic etiology of [neurodevelopmental disorders] enhances our understanding of disease mechanisms, but it usually requires access to the developmental processes of the human brain. Brain organoids recapitulate early brain development and generate diverse cell types found in vivo," the team write in their paper.
While the organoids contain several tissue types that make up our brain, their models lacked some common brain tissues like microglia cells so remain an incomplete model, so these will still need to be tested, the team cautions.
This new technique will bring about significant changes in genetic screening, and since organoid technology can be applied to many different human tissues, the team believes it will rapidly progress our grasp of genetic diseases.
"The ability to determine cell type-specific contributions to genetic disorders in a systematic, scalable, and efficient manner will greatly enhance our understanding of disease mechanisms," they write.
"As the CHOOSE system provides a robust, precisely controlled screening strategy, we anticipate that it will be widely applied beyond brain organoids to study disease-associated genes."
And with this, the secrets of the human brain are closer than ever to being understood.
This research was published in Nature.