Microscopic backpacks full of drugs designed to stick to troublesome immune cells have been shown to improve the health of mice with a condition similar to multiple sclerosis in humans.
Developed by researchers at Harvard University in the US, the microparticle luggage could replace existing therapies, taking advantage of the tendency for errant myeloid immune cells to venture into tissues where they would cause inflammation, instead encouraging repair.
Multiple sclerosis (MS) is a particularly debilitating autoimmune disease that causes the insulating myelin around nerves to deteriorate, disrupting brain-body communication and making it harder for those with the condition to move and function.
Although MS has no cure, drugs that target reactive immune cells or reduce inflammation can change its course. Unfortunately many of these treatments come with significant risks, from depression and infections to problems with thyroid and other organs.
"Despite the key role of myeloid cells in the pathophysiology of MS, current treatments do not specifically target myeloid cells or directly make their use for modulating the disease," the authors note in their published paper.
To turn the potentially inflammatory white cells into tiny paradmedics, the researchers took a type of myeloid cell from healthy mice and grew them outside of their bodies. They then attached microscopic disk-shaped objects carrying microparticles of drugs to the cells' surfaces, effectively giving them a tiny knapsack of medicine.
When the backpack-carrying cells were put into a mouse model of MS, they were able to significantly improve immune responses specific to the paralytic condition, partially reversing paralysis and enhancing motor functions.
"These are very plastic cells that can toggle between different states and are thus hard to control. Our biomaterial-based backpack approach is a highly effective way to keep them locked into their anti-inflammatory state," explains senior author Samir Mitragotri, a bioengineer at Harvard University and the Wyss Institute.
Myeloid cells of the innate immune system sparking inflammation in the central nervous system (CNS) is a major factor in MS. These inflamed lesions then draw in more myeloid cells, as well as T and B cells from the adaptive immune system, causing damage to the myelin coating surrounding nerves.
Mitragotri and colleagues had already created a therapy for fighting tumors that uses backpack-loaded macrophages, a type of myeloid cell. By putting certain molecules in the backpacks, the team was able to control how the cells behaved. The backpacks stayed on the surface of the macrophages even as other cells rapidly absorbed and deactivated the material.
Monocytes – a type of myeloid cell – are the parent cells of macrophages. They develop in bone marrow and can find their way into brain tissue before differentiating into macrophages, which are one of the most common inflammatory cell types in MS lesions.
This time the team attached backpacks to monocytes, filled with interleukin-4 and dexamethasone molecules that work together to boost anti-inflammatory and regulatory functions and slow down pro-inflammatory functions.
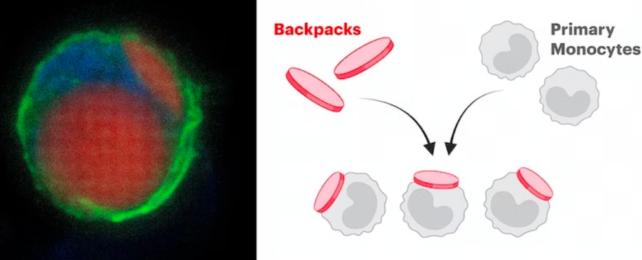
"Because of their ability to invade the CNS, infiltrate inflammatory lesions, and differentiate into macrophages, a backpack strategy allowing control over monocyte differentiation made extreme sense," says chemical and biomedical engineer Neha Kapate.
"They also reduced inflammation inside the lesions and shifted the local and systemic MS-associated immune response towards a therapeutic outcome."
The scientists found the mouse models showed major improvement when treated with the monocyte cells that hiked in carrying special backpacks. After treatment, those mice only had a minor limpness in their tail, while the mice that didn't get the treatment were completely paralyzed in their back legs. The treated mice also lived longer than the untreated mice.
The team wants to do more research on this technique to see if it works on a more common relapsing form of MS, as the mouse models only mimic a progressive form of MS. If it does work on the relapsing form, it could be a big help for many people with MS by preventing inflammation early on.
"Disease treatment could be improved in combination with other medications that target the adaptive immune system," the authors write. The symptoms of MS manifest in different ways depending on where the lesions are in the nervous system. Autonomic, visual, motor, and sensory difficulties are the most common, but a person can have just about any neurological symptom, all of them impacting quality of life.
While the basic mechanism is understood, the exact triggers of MS are unclear. It's thought to be caused by various environmental factors with research linking it to childhood trauma and viral infection.
Since 2013, the number of people with MS has increased by 30 percent, and around 2.8 million people globally are suffering with the disease, so finding ways to mitigate this is an important area of research.
The study has been published in the journal PNAS.