Encased inside some of the oldest rocks on Earth are previously overlooked nanocrystals that tell a story about how life might have emerged.
Earth scientists at the University of Western Australia and the University of Cambridge say their findings could explain why phosphorus became a major building block of life and how molecules first clicked together to form primitive RNA at hydrothermal vents on the seafloor.
They examined 3.5-billion-year-old rocks from the Pilbara region of Western Australia under a transmission electron microscope and found unexpected minerals.
The Pilbara is renowned for its pristine preservation of the Earth's crust during the Archean era when life was just getting started. The rocks in this area are a time capsule containing insights into prebiotic chemistry.

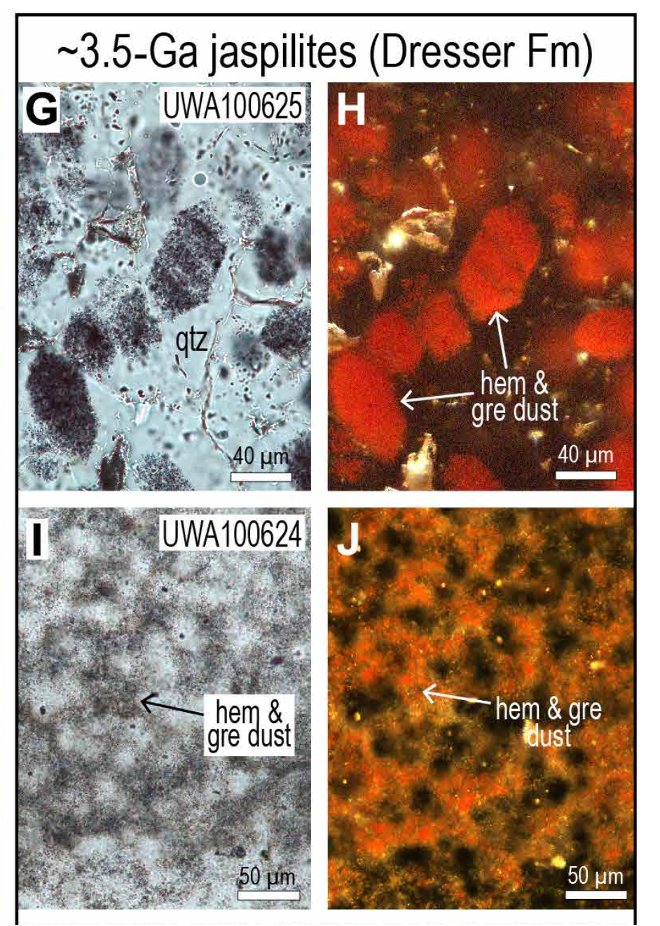
From afar, a trained eye might identify the Pilbara's stripy red rock as a mix of very fine quartz (containing silicon and oxygen) and hematite (made of iron and oxygen), a combination known as jaspilite.
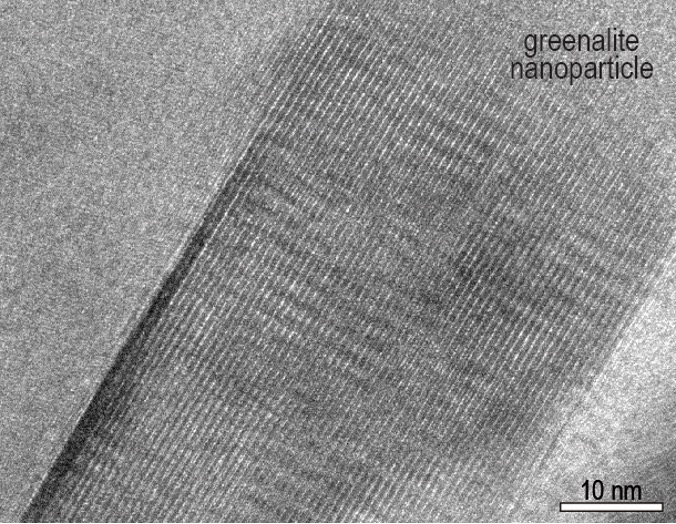
Closer inspection reveals something surprising; hidden nanocrystals with interesting properties. Dispersed throughout the jasper beds are fine particles of greenalite, a mineral containing iron, silicon, and oxygen, which would have been ejected from a nearby hydrothermal vent and precipitated on the seafloor billions of years ago.
"We found hidden between the more conspicuous iron oxides (which gives the rock its bright red color) much more abundant iron clays," University of Western Australia geologist Birger Rasmussen told ScienceAlert.
"It's amazing you can see nanoparticles in rocks so old, and part of the reason for that is they're sealed in these relatively chemically inert materials."
At the nanoscale, the structure of greenalite is unusual. Particle edges are corrugated due to a misalignment in its crystal structure between the iron-rich octahedral layers and the silica-rich tetrahedral layers.
"It produces a series of parallel grooves on the edges that are the perfect size for things like RNA and DNA," says Rasmussen, explaining this makes the clay nanoparticles the perfect catalytic tool for aligning the components of these biomolecules so they can easily click together.
Billions of years ago, hydrothermal vents might have produced trillions of microscopic clay particles with grooves that acted like assembly lines, concentrating RNA or pre-RNA.
Long considered a likely place for life to emerge, hydrothermal vents provide the perfect location for this process to happen. They constantly churn seawater through magma chambers, and spew hot, smoky plumes filled with nutrients back into the ocean.
"It's a great place for chemical reactions to occur … because they are areas of extreme gradients," says Rasmussen.
The 3.5-billion-year-old rocks from the Pilbara also contained nanoparticles of fluorapatite (a mineral made of oxygen, calcium, fluorine and phosphorus).
Scientists have been puzzled over why phosphorus is found in so many biological structures (including DNA, membranes, and lipids) in spite of such low concentrations of the element in the ocean.
But the presence of the phosphorus-containing mineral fluorapatite in billion-year-old rocks provides a potential explanation: hydrothermal vents might have been an early source of accessible phosphorus.
The researchers' modeling suggests that the concentration of phosphorus in deep seawater 3.5 billion years ago was likely 10 to 100 times higher than it is today.
"Why did life select phosphorus for so many essential biochemical processes, including the manufacture of genetic material, when it is so scarce in the ocean today? The answer may be that phosphorus was much more abundant during the origin and early evolution of life," says Rasmussen.
This paper was published in Science Advances.
