A common genus of microbe found in wet, boggy environments could play a key role in the development of Parkinson's disease, by excreting compounds that trigger proteins inside brain cells to form toxic clumps.
The findings, made by a small team of researchers at the University of Helsinki and the University of Eastern Finland, build on the results of an earlier investigation showing that the severity of the neurodegenerative disorder in volunteers increased with concentrations of Desulfovibrio bacterial strains in their feces.
By now demonstrating a potential path from the presence of the bacteria in genetically edited worms to physical changes in the brain that coincide with Parkinson's disease, the researchers hope to one day improve early diagnosis of the disease in humans, or even slow its progress.
"Our findings make it possible to screen for the carriers of these harmful Desulfovibrio bacteria," says senior author Per Saris, a microbiologist at the University of Helsinki in Finland.
"Consequently, they can be targeted by measures to remove these strains from the gut, potentially alleviating and slowing the symptoms of patients with Parkinson's disease."
Ever since the English physician James Parkinson first described the disease as a neurological condition some two centuries ago, researchers have sought an explanation for why some people develop a drastic loss of fine motor control as they age.
Physiologically speaking, small inclusions known as Lewy bodies accumulate in the cells of specific regions of the brain of individuals diagnosed with Parkinson's disease.
More recently, investigations of these microscopic clumps of material have revealed them to largely consist of a type of protein called α-synuclein, which is typically involved in the release of neurotransmitters.
Just how this clumping contributes to the pathology of Parkinson's still isn't entirely clear, though it's suspected the very presence of these concentrations, called protofibrils, can't be great for the healthy functioning of nerve cells.
What is also something of a mystery is the initial cause of α-synuclein's aggregation. Though Parkinson's can run in families, genetics only seems to explain around 10 to 15 percent of all cases.
That leaves environmental conditions as a probable suspect, with studies finding the kinds of bacteria we harbor in our guts predicting the likelihood of an individual having, or at least developing, Parkinson's symptoms.
With Saris's 2021 study, there was finally evidence of a single prime suspect researchers could focus on.
"The disease is primarily caused by environmental factors, that is, environmental exposure to the Desulfovibrio bacterial strains that cause Parkinson's disease," says Saris.
In the new study, Saris and his team took fecal samples from 10 patients with Parkinson's disease and their healthy spouses, and isolated any strains of Desulfovibrio present.
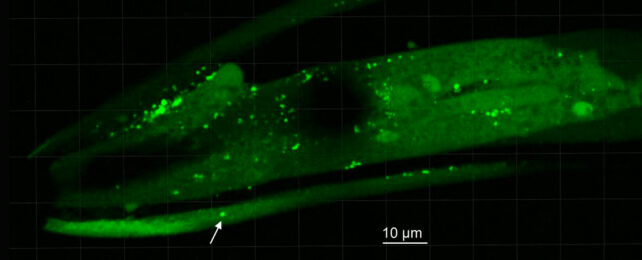
Along with two different control groups of bacteria belonging to a completely different genus, the extracted test microbes were then fed to transgenic specimens of Caenorhabditis elegans nematode, which had been modified to express human α-synuclein.
A statistical analysis based on microscopic observations of the nematodes' heads revealed those fed Desulfovibrio were indeed far more likely to produce α-synuclein clumps, and those clumps were more likely to be much larger.
Tellingly, Desulfovibrio strains collected from Parkinson's patients were also better at aggregating the proteins in C. elegans than those collected from their partners.
What's more, those worms typically died in larger numbers than those in control groups.
Of course, there's a world of difference between worms and humans. While the same experiment could never be replicated in a sample of healthy people, studies will continue to look closely at ways Desulfovibrio in our own guts might spark the formation of α-synuclein aggregates that could migrate through the body.
In time, we may even be able to manage the progress of Parkinson's disease using therapies that target the digestive system and its surrounding nerves, instead of the brain.
"Once the Desulfovibrio bacteria are eliminated from the gut, α-synuclein aggregates are no longer formed in intestinal cells, from which they travel towards the brain via the vagus nerve like prion proteins," Saris suggests.
This research was published in Frontiers in Cellular and Infection Microbiology.