A small tweak on a definitive experiment in quantum physics has allowed scientists to observe for the first time exactly how molecules behave as waves.
The results are solidly in line with what theory covering complex quantum phenomena predicts, so don't expect any radical new physics here. But as with most quantum experiments, the implications of seeing such a counter-intuitive theory in action makes our head spin.
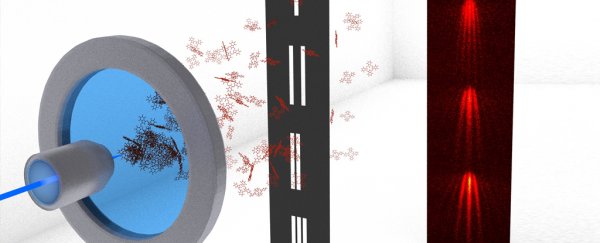
Researchers from the Universities of Vienna and Tel Aviv have recently collaborated on turning a two-decade old idea into a reality, replacing tiny particles with large organic molecules in a variation on Clinton Davisson and Lester Germer's classic 1927 double slit experiment in order to test the limits of a law governing their behaviour.
"The idea has been known for more than twenty years," says researcher Christian Brand from the Vienna Centre for Quantum Science and Technology at the University of Vienna.
"But only now do we have the technological means to bring all the components together and build an experiment capable of testing it with massive molecules."
To understand the significance, it helps to go back to the beginning.
For the first quarter of the 20th century, scientists were wrestling with what seemed like two completely different Universes of physical laws.
One was the Universe of Newton, where falling apples and shooting stars behaved in similar ways, only differing in terms of scale.
The second was born when Albert Einstein suggested that the mathematics being invented to explain how light was absorbed and emitted wasn't just a convenient way to crunch the numbers – light really was made up of discrete bits called quanta.
Enter stage left, Prince Louis de Broglie.
Because the idea of light being made of tiny shooting balls wasn't messed up enough, this intrepid French physicist decided one way to make sense of the latest models of the atom was to describe electrons – those little spheres whizzing around a nucleus – as waves as well.
Famous names such as Werner Heisenberg and Erwin Schrödinger subsequently found different ways to predict how an atom's structure should behave, but one pictured electrons as continuous waves and the other as discrete bits of stuff.
The mad thing was, both theories were solid. By the same token, a thing couldn't be a wave and a ball at the same time, could it?
American physicists Clinton Davisson and Lester Germer then took inspiration from an even earlier experiment that had demonstrated light was a wave.
Their version showed that a beam of electrons passing through a pair of closely-aligned parallel slits could produce a wave-like pattern of behaviour similar to light, backing up de Broglie's hypothesis. Case closed.
Except ever since then, various versions of this double slit experiment have continued to mess with our minds, showing small objects like electrons and photons can behave as both particles and waves, depending on how we measure them.
Worse still, it's not just a matter of the very tiny. In 2012, a new record was set in showing a molecule a whopping 800 atoms in size also has wave-like properties.
This latest experiment hasn't smashed any records, but the researchers still used massive free-floating particles that weighed 515 atomic mass units, or roughly 42 carbon atoms in size. Not exactly tiny, and not easy to manage.
Their goal was to put some limits on the wave-like nature of big things like molecules by passing them through different numbers of slots.
It's tempting to picture those waves as bunches of spheres jittering up and down like fleas on a hotplate.
Instead, an object such as an electron, a photon, a molecule, or (just to blow your mind) your grandmother, can be thought of as a blend of properties called a superposition that have different states at once.
The probabilities of those states, each describing its position and energy in time and space, is what we call waves. Seriously, stop trying to imagine it in a classical, physical sense, you'll get a nose bleed.
For tiny particles, this probability can be inferred from measurements plugged into something called Born's law.
More complex systems, such as molecules (and presumably grandmothers), demand extensions to this formula.
A little over 20 years ago a physicist named Rafael Sorkin determined you only needed the measurements from just two paths – such as those taken through dual slits – for certain extensions to Born's law to still work. Adding a third, fourth, or hundredth should make no difference.
Thanks to the results of this experiment, we can sleep easier at night knowing Sorkin's 'two pathway' limit stands for molecule-sized particles.
"This is the first time an explicit test of this kind has been conducted with massive particles", says researcher Joseph Cotter the University of Vienna.
"Previous tests have pushed the frontiers with single photons and microwaves. In our experiment, we put bounds on higher-order interference of massive objects."
While this is all well and good for physics, it's also one more piece of evidence that shows quantum mechanic weirdness, such as existing as both particles and waves, isn't just something that happens to unimaginably small things.
No wonder our head feels fuzzy.
This research was published in Science Advances.