A drug that's currently in development for treating leukemia has delivered a surprise in mouse studies by reversing a previously untreatable symptom of schizophrenia - the impairment of spatial working memory.
In humans, schizophrenia has proved extremely difficult to understand, let alone treat. The neuropsychiatric disorder has a range of symptoms affecting the person's mood, behaviour, and ability to function. Cognitive impairment, which can include trouble with memory, is thought to be a core feature of schizophrenia.
Nevertheless, to this day, there are no standardised treatments for treating working memory impairments in schizophrenia, neither therapeutic nor pharmaceutical. The drugs we currently have for schizophrenia can largely control psychotic symptoms, not cognitive ones.
Researchers at Columbia University now think there might be a way to treat schizophrenia-associated working memory deficits after all.
Using a mouse model, the team successfully reversed a mutation in the gene SETD1A, which they had previously associated with the disorder; mutations to this particular gene "confer a large increase in disease risk", the team writes in their paper.
"We were surprised to see that restoration of SETD1A activity in the brain of adult mice restores their learning," neuroscientist Joseph Gogos from Columbia's Zuckerman Institute told ScienceAlert, "which means the damage done by the mutation during brain development is not irreversible."
The results are a promising step forward, Gogos says, as "a way to use knowledge from genetic studies to identify drugs that restore normal cognitive and cellular function in the adult brain after the onset of disease."
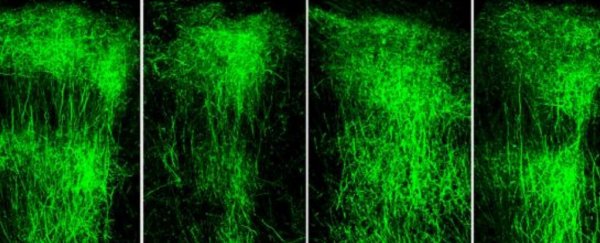
While mice don't get schizophrenia, the animals with this genetic mutation did show signs of spatial memory deficit, like being unable to navigate a simple maze. Not only that, but their prefrontal cortex looked markedly different to those mice without the mutation, revealing much shorter neurons that look as though their branches had been stunted.
Fixing these cells would require a drug of some sort that could manipulate the SETD1A gene. The problem was, no such drug existed, so Gogos and his colleagues had to get clever.
In their digging, they found that when another gene, called LSD1, was switched off, SETD1A's harmful effects disappeared. Repurposing the LSD1 inhibitor drug seemed like a logical shot.
"Within a few weeks of administering an LSD1 inhibitor, the animals' memory improved dramatically," says neuroscientist Jun Mukai.
"Even more striking was what we observed in the animals' brains: their axons grew in similar patterns to what we see in a healthy mouse brain."
Better yet, the authors say this drug was acting on the underlying mechanisms that drive the memory deficits, rather than just targeting the symptoms themselves.
"We've found definitive proof that not only does [SETD1A] guide early development, but it also supports ongoing functions in the adult brain, such as axonal growth," claims neuroscientist Enrico Cannavó.
The team now hopes to use human genetic studies to understand disease mechanisms in model organisms like mice, so they can identify pathways for future drugs.
"These are animal models of mutations that we know (unequivocally) increase risk of schizophrenia in humans," Gogos told ScienceAlert.
"By analysing how these mutations affect the mouse brains, we can infer how they may affect the development and function of the human brain."
The research was published in Neuron.