Researchers have uncovered what they say is a new class of ultra-low-density ice, which crystallises amid extreme negative pressure on water molecules.
While many of us are only familiar with the kind of frozen water that keeps our drinks nice and chilled, regular ice on Earth is just one of around 20 known phases of ice – and the new forms discovered by researchers in Japan appear to have the lowest density of all known ice crystals.
The new ice is called aeroice, and its discovery by researchers at Okayama University is part of an emerging wave of research into how water freezes.
A lot of previous studies have seen huge amounts of pressure applied to water molecules to create kinds of ultra-dense ice that don't naturally occur on Earth under ordinary atmospheric conditions, but here the team was focussed on the opposite cause and effect – the absence of pressure to make ice that isn't dense.
"Our research, which surveys an entire negative-pressure region for the first time, provides a significant stepping stone in exploring this vast and intricate territory on the phase diagram," says lead researcher Masakazu Matsumoto.
As it stands, there are 17 recognised solid crystalline phases of water that can form all different kinds of ice. Of these, only two occur naturally on Earth, hexagonal ice and cubic ice.
It's the former, known as Ice Ih, that makes up almost all the ice on our planet, but another type called Ice Ic can form in Earth's upper atmosphere.
All the other kinds of ice phases are what happens when water molecules are frozen in extreme conditions – often involving severe variations in pressure to replicate how ice might form in exotic or far-flung environments, such as when icy planetary bodies collide in space.
Anyway, of the 17 known ice phases – which are named in order of their discovery – only two have lower density than normal ice. These are the latest additions to the lineup, called Ice XVI and Ice XVII.
Ice XVI was discovered in 2014 by researchers who found ice could form in a kind of 3D crystalline cage called a zeolite structure.
In the right conditions, this frozen cage could take shape around a guest molecule – in this case, neon atoms – which could then be extracted from the structure, resulting in what became the lowest density phase of ice yet discovered.
 Masakazu Matsumoto
Masakazu Matsumoto
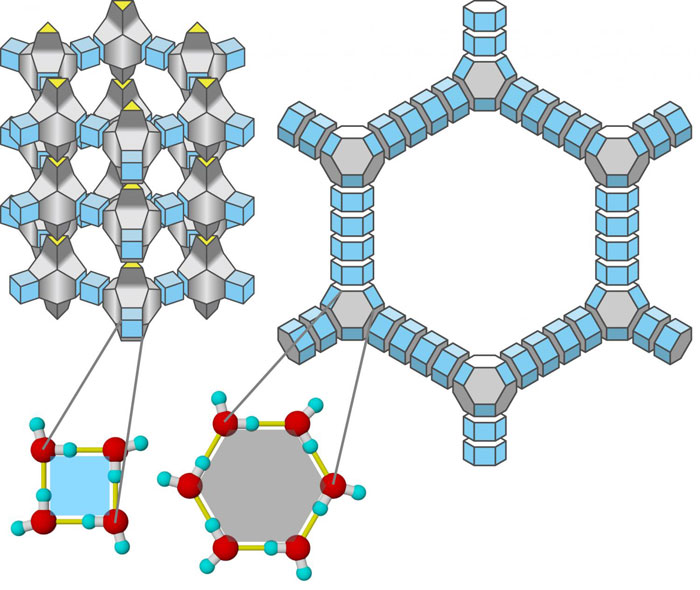
Above, you can see an example of zeolitic ice on the left, with the molecular structure of one of the types of aeroice on the right.
Not to be outdone by Ice XVI, simulations by another team of researchers surpassed the milestone in 2016 with Ice XVII, using a similar molecule trapping-and-extracting technique, that theoretically results in ice with 25 percent less density than Ice XVI.
The new discovery by Matsumoto's team, aeroice, again stems from molecular rearrangements conducted at negative pressure, but this time involving silica (aka silicon dioxide, SiO2).
In simulations, the team removed the two oxygen atoms from SiO2 molecular structure and then swapped out each molecule's single silicon atom for a single oxygen atom, before adding hydrogen atoms.
The end result produces a kind of ice with a density about half that of liquid water (~0.5 g/cm3), but despite that extreme low-density, the researchers say aeroices are more stable than any other kinds of zeolite ice that have been engineered to date.
Additional simulations suggest aeroices could become even less dense – between 0 and 0.5 grams per cubic centimetre – with additional tampering.
By adding polyhedral building blocks (structures with six planes or more), the molecules could maintain their crystalline stability while making the overall structure sparser, which would lower the density – meaning any number of aeroices could ultimately be possible.
"Ices with lower density than normal ice are also found to be manifold," says Matsumoto.
"These new structures are the aeroices, and they can be more stable than any zeolitic ice at certain thermodynamic conditions under negative pressure."
While the findings may be largely of academic interest right now, the potential applications of discoveries like this are huge, ranging from understanding how water behaves in nanotubes and nanopores, to discovering how ice might behave for off-world colonists exploring the far reaches of the Solar System.
That's a lot to think about, sure, and it'd probably go down better with a cool beverage in your hand.
The findings are reported in The Journal of Chemical Physics.
