Scientists have discovered how our DNA can use a genetic fast-forward button to make new genes for quick adaptation to our ever-changing environments.
During an investigation into DNA replication errors, researchers from Finland's University of Helsinki found that certain single mutations produce palindromes, which read the same backward and forward. Under the right circumstances, these can evolve into microRNA (miRNA) genes.
These tiny, simple genes play a significant role in regulating other genes. Many miRNA genes have been around for a long time in evolutionary history, but scientists discovered that in some animal groups, like primates, brand-new miRNA genes suddenly appear.
"The emergence of new genes from nothing has fascinated researchers," says bioinformatician Heli Mönttinen, first author of the new study.
"We now have an elegant model for the evolution of RNA genes."
The errors that allow this remarkably efficient method of gene creation are called template-switching mutations (TSMs). The TSM-associated process of miRNA creation is much faster than how new functional proteins evolve.
"DNA is copied one base at a time, and typically mutations are erroneous single bases, like mis-punches on a laptop keyboard," says project leader and bioinformatician Ari Löytynoja.
"We studied a mechanism creating larger errors, like copy-pasting text from another context. We were especially interested in cases that copied the text backward so that it creates a palindrome."
All RNA molecules need repeating sets of bases that lock the molecule into its working shape. The team chose to concentrate on microRNA genes, which are extremely short, consisting of around 22 base pairs.
But even for simple microRNA genes, the chances of random base mutations slowly forming these kinds of palindromic runs are very low.
Scientists have been puzzled by where these palindromic sequences came from. It turns out TSMs can rapidly produce full DNA palindromes, making new microRNA genes from previously noncoding DNA sequences.
"In an RNA molecule, the bases of adjacent palindromes can pair and form structures resembling a hairpin. Such structures are crucial for the function of the RNA molecules," says biotechnologist Mikko Frilander.
The complete genomes of many primates and mammals have already been mapped out. By comparing these genomes using a custom computer algorithm, the researchers were able to find out which species have the microRNA palindrome pair.
"With a detailed modeling of the history, we could see that whole palindromes are created by single mutation events," Mönttinen explains.
The diagram below illustrates the process nicely. As DNA replication begins running through each base pair on its recipe list, it stops when it hits a mutation or faulty base pair.
Replication then jumps to the adjacent template and begins replicating those instructions but backwards.
When replication returns to the original template this creates a tiny little palindrome that can pair with itself in a hairpin structure.
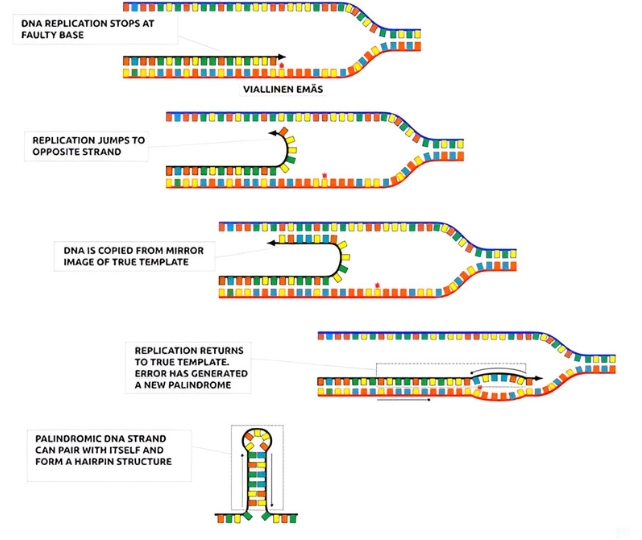
Template switching during DNA replication allows a single mutation event to create the perfect structure in the DNA for a new miRNA gene. This is far more efficient than the slow and gradual changes that can occur with individual building blocks.
In the primate family tree, over 6,000 of these structures were found, which could have given rise to at least 18 brand-new miRNA genes in humans. That's 26 percent of all the miRNAs that are thought to have emerged since primates first appeared.
Findings like these, which span evolutionary lines, point to a universal miRNA gene creation mechanism, and the team thinks that the results can be applied to other RNA genes and molecules as well.
It appears relatively easy for new microRNA genes to appear that could potentially impact human health. Some TSM-associated miRNAs have already shown functional significance, like hsa-mir-576 which influences the antiviral response in primates.
"Many TSM variants capable of becoming miRNA genes segregate among the human populations," the authors write, "indicating that the TSM process is active and shaping our genomes right now."
The study has been published in Proceedings of the National Academy of Sciences.