Scientists are always on the lookout for new materials that can enable improved energy storage and quicker energy transfers, and a new study suggests what could be a dramatically simple approach for achieving those ends: just add water.
By adding atomically thin, nanoscale layers of water to an existing material, researchers found it was able to store and deliver energy more quickly than the same material without the water layers, which could lead to new ways of manufacturing better batteries and improved electric devices.
"This is a proof of concept, but the idea of using water or other solvents to 'tune' the transport of ions in a layered material is very exciting," says one of the team, materials scientist Veronica Augustyn from North Carolina State University.
"The fundamental idea is that this could allow an increased amount of energy to be stored per unit of volume, faster diffusion of ions through the material, and faster charge transfer."
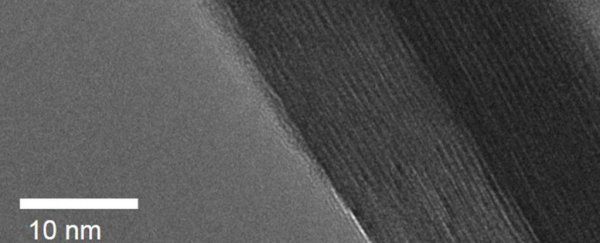
Augustyn's team compared two materials in their research: a crystalline tungsten oxide, and the same material in a layered from – called crystalline tungsten oxide hydrate – which was interspersed with extremely thin layers of water (seen as stripes in the image below):
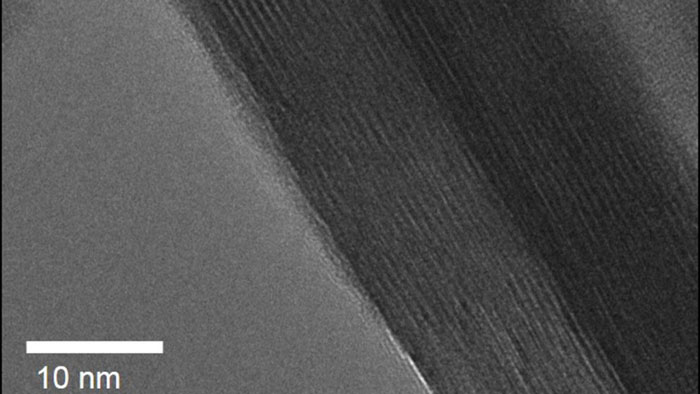 North Carolina State University
North Carolina State University
The idea is to enable fast diffusion of ions in a solid-state structure, using water to speed up the transfer of energy throughout the medium, while still retaining the ability of the material to store as much energy as possible.
Research in this field – called pseudocapacitance – has gone on for decades, but researchers are now better able to explore their hypotheses thanks to advances in materials science and nanostructuring methods.
"The goal for many energy-storage researchers is to create technologies that have the high energy density of batteries and the high power of capacitors," says one of the researchers, James Mitchell.
"Pseudocapacitors like the one we discuss in the paper may allow us to develop technologies that bridge that gap."
In testing with the hydrate material, the team found that it was able to store more energy than the regular tungsten oxide, but only when it was charged for short periods.
After being charged for 12 seconds, the water layer oxide stored more energy, but when the charging cycle was extended to 10 minutes, the regular oxide stored more – although the hydrate stored energy more efficiently than the conventional material, by wasting less energy as heat.
The team says their work is only experimental at this stage, so we probably won't be seeing nanoscale water layers embedded in our personal electronics just yet.
But in the long term, now that we know that hydrate layers can confer benefits in energy storage and transfer mechanisms, it means manufacturers could end up using them in all kinds of electric devices in the future.
"Again, this is only a first step, but this line of investigation could ultimately lead to things like thinner batteries, faster storage for renewable-based power grids, or faster acceleration in electric vehicles," says Augustyn.
"We are now moving forward with National Science Foundation-funded work on how to fine-tune this so-called 'interlayer,' which will hopefully advance our understanding of these materials and get us closer to next-generation energy-storage devices."
The findings are reported in Chemistry of Materials.