For the first time, scientists have successfully regrown part of the spinal cord responsible for voluntary movement in mice, using patches of stem cells. While we're still a long way from a cure for paralysis and other spinal cord injuries in humans, the success of the experiment goes against what researchers had assumed for many years - that you can't regenerate neurons in the spinal cord.
The set of cells in question are the corticospinal axons, thought to be the most important motor system in humans. "It has not been successfully generated before," said one of the team, Mark Tuszynski from the University of California, San Diego. "Many have tried, many have failed - including us, in previous efforts."
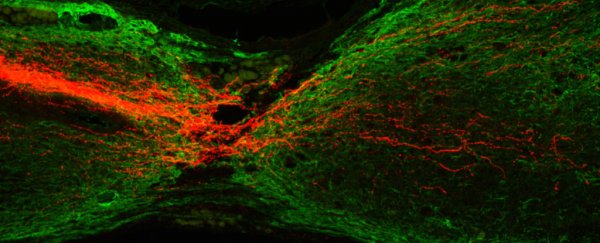
By grafting stem cells into the injured rats and specifically directing them to develop as spinal cord cells, forelimb movement was improved in the animals - the partially paralysed rats were once again able to reach out and grab treats. The results go against an existing belief that corticospinal neurons lack the internal mechanisms to be able to regenerate in this way.
"The new thing here was that we used neural stem cells for the first time to determine whether they, unlike any other cell type tested, would support regeneration," says Tuszynski. "And to our surprise, they did."
In humans, the corticospinal tract extends from the cerebral cortex in the upper brain down into the spinal cord, and Tuszynski and his colleagues think that the same therapy could eventually be adapted to work beyond rats. But extensive testing will be required to prove the procedure's long-term safety and reliability before human trials are even considered, and then there's the question of identifying the best type of human neural stem cell to use.
For the purposes of this study, rat and human neural progenitor cells were used, because they can produce several different types of cells found in the nervous system depending on the chemical signals given to them as triggers. The scientists found that the injected cells took root, filled damaged areas with tissue and corticospinal axons, and connected with severed nerves that then allowed the brain signals to flow again.
We've seen previous work in this area with rats, but this is the first time these specific cells have been targeted and healed, and that increases the likelihood that the same process will eventually work in human beings.
"Now that we can regenerate the most important motor system for humans, I think that the potential for translation is more promising," says Tuszynski.
The research has been published in Nature Medicine.