A team of chemists from Osaka University in Japan have identified a rare property in a crystal. When exposed to the cool glow of ultraviolet (UV) light, the solid organic material transforms into a liquid.
What's more, this crystal undergoes an interesting series of changes in its luminescence as it melts that point to changes in the structure of the crystal at a molecular level.
Though unusual, it's not the first substance found to undergo what's known as photo-induced crystal-to-liquid transition (PCLT). But being able to study the transition using light could help scientists understand it better, potentially opening up a whole range of potential uses in photonics, electronics, and drug delivery.

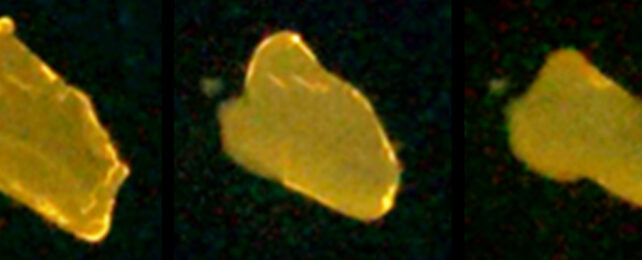
"This is the first organic crystal we know of that exhibits a luminescent evolution during crystal melting, showing changes in intensity and color, from green to yellow," says chemist Mao Komura.
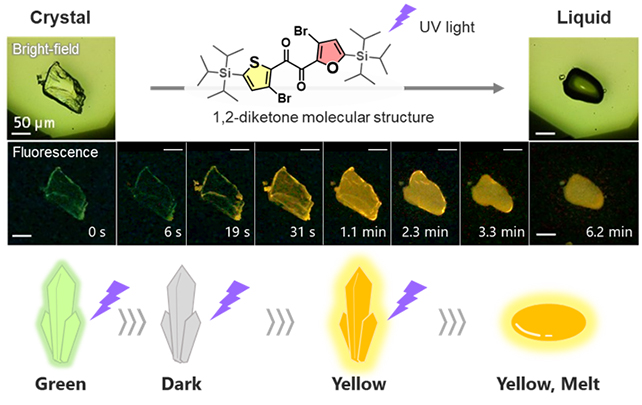
The material is a type of organic compound known as a heteroaromatic diketone, one the team dubbed 'SO' after the sulfur and oxygen in its two rings.
When first exposed to UV light, the SO crystal compound glows in a faint green light. As the exposure continues, however, it glows yellow and slowly melts. Based on close observations of the sharpness of the boundary between the states, it's clear heating isn't responsible for the transition.
Using theoretical calculations and a variety of study techniques (including X-ray analysis and thermodynamic property analysis), plus data from previous research, the team determined that diketone SO was actually switching from one molecular form ("skew") to another ("planar").
Further insights were obtained from other similar crystal compounds, that either didn't melt or did melt but didn't change color. That tells the researchers something about the molecular changes that occur as these crystals shift from solids to liquids.
"We found that the changes in luminescence arise from sequential processes of crystal loosening and conformational changes prior to melting," says chemist Yosuke Tani, from Osaka University.
Working backwards, it shows that there's something special about the molecular arrangement of these materials that means they'll melt and switch between phases when exposed to certain types of light.
And being able to control materials with light could be very useful: it's relatively affordable and simple to do, environmentally friendly, and non-invasive. One example application suggested by the researchers is a reversible adhesive that can be modified through light exposure.
What's key to the progress outlined in this study is the way that diketone SO changed color, giving the researchers a vital insight into what was going on at the smallest scales inside the crystal compound.
"These visual indications of the steps of the PCLT process enabled us to advance the current understanding of crystal melting at the molecular level," says Tani.
The research has been published in Chemical Science.