Water may seem boring, but it's way weirder than it looks. Scientists in Japan have now shown that when confined to tight spaces, water molecules can behave like both a solid and a liquid simultaneously.
The differences between liquid water and ice that we experience at the macro scale start at the micro. In ice, molecules are locked into rigid structures, while in water, they essentially float freely, constantly forming and breaking bonds.
Related: Water Can Separate Into 2 Different Liquids. We Just Got Closer to Knowing Why
In the peculiar state described in the new paper, the molecules kind of do both. They're in a fixed position, like ice, but spin quickly as they would in liquid. Known as the premelting state, this condition has previously eluded direct study.
"The premelting state involves the melting of incompletely hydrogen-bonded H2O before the completely frozen ice structure starts melting during the heating process," says Makoto Tadokoro, a chemist at Tokyo University of Science.
"It essentially constitutes a novel phase of water in which frozen H2O layers and slowly moving H2O coexist."
Seeing this strange state required a complex experimental setup. For starters, the water wasn't quite the type we're used to in everyday life: it's what's known as 'heavy water', where the hydrogen atoms are swapped out for deuterium, an isotope of hydrogen that packs a neutron in its nucleus.
This "D2O" was then confined to an extremely tight space, where all kinds of exotic behaviors emerge. The researchers made rod-shaped crystals with tiny, hydrophilic channels just 1.6 nanometers wide, froze the heavy water in them, and slowly warmed them back up.
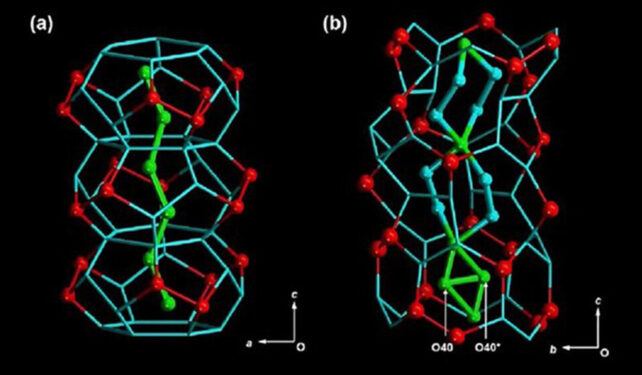
Finally, they watched the whole process using static solid-state deuterium nuclear magnetic resonance (NMR) spectroscopy. This revealed that the molecules were forming a hierarchical, three-layered structure, with different types of movements and interactions in each layer.
The premelting state is probably most familiar to us as a thin film of water that forms on the surface of ice, even if temperatures are still below freezing. But it happens in a different way in that bulk ice than it does under extreme confinement.
Water is already known to do some weird stuff when confined to the nanoscale. Its electrical properties can change; it can be made 'unfreezable' even at temperatures approaching absolute zero, or it can freeze solid at temperatures that should have it boiling.
Tapping into these quirks could have practical uses too, the team says.
"By creating new ice network structures, it may be possible to store energetic gases such as hydrogen and methane and develop water-based materials such as artificial gas hydrates," says Tadokoro.
The research was published in the Journal of the American Chemical Society.

