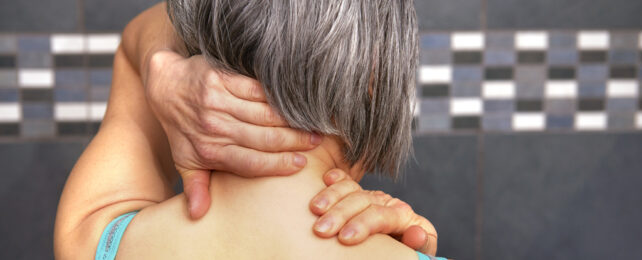
Roughly one in five adults in the US experience pain that persists relentlessly for months on end. Yet, the only effective treatment we have so far involves painkillers, which can be addictive and rarely sufficient.
To better understand the underlying mechanisms of chronic pain, University of Pennsylvania neuroscientist Mayank Gautam and colleagues honed in the tendency for episodes to be triggered by a mere touch.
Touch is detected by a series of mechanoreceptors in our bodies. Some of them activate with a slight pressure, whereas others require much firmer impact to engage.
A combination of pain neurons (nociceptors) and touch neurons help our bodies decide if something is uncomfortable or not. The links between these two processes is what allows us to disrupt a pain signal by, for example, rubbing a sore spot.
Gautam and his team used a combination of light-triggered genetic tools and high speed imaging to examine how one of these fast-acting and most sensitive mechanoreceptors, Aβ-LTMRs, works in mice.
When the researchers deactivated Aβ-LTMRs, mice showed a decreased response to gentle touch as predicted. But this also created a greater response in the pain neurons and central nervous systems of mice that had been given a treatment that made them experience chronic inflammation.
This indicates that as well as sensing touch and contributing to local pain detection Aβ-LTMRs also play a role in mediating pain response across the entire body in the presence of inflammation.
When the team intentionally activated Aβ-LTMRs in mice with both inflammation and functioning receptors, the test animals responded in a way that indicates they experienced localized pain. Activating the same receptors in a more centralized part of the nervous system called the dorsal column, however, reduced pain in these mice.
Together these results reveal Aβ-LTMRs contribute to the sensation of touch-triggered pain locally but also help subdue it on a global scale.
This could explain why activating receptors away from the injury site through electrical nerve stimulations can soothe pain.
"Additional real-life therapy procedures, including massage therapy and electroacupuncture, presumably involve the activation of Aβ-LTMRs for their beneficial effects," the researchers suspect.
In more extreme cases, chronic pain can limit individual's capacity to work or even do many of life's most basic tasks, such as eating and sleeping. It chemically disrupts the ability to regulate emotions, resulting in further stress, guilt, sleeplessness and even personality changes.
Figuring out how all the different processes work together to signal pain in mammalian bodies brings us closer to finding safer, more reliable relief.
This research was published in Nature Communications.