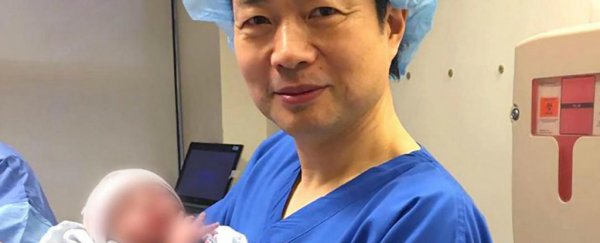
A five-month-old boy is the first baby to be born using a controversial new 'three-parent' technique.
That means this baby contains DNA from three parents - something which in this case allowed him to avoid having a deadly genetic condition passed down by his mother.
"This is great news and a huge deal," researcher Dusko Ilic from King's College London, who wasn't involved in the project, told Jessica Hamzelou at New Scientist in an exclusive. "It's revolutionary".
The technique - which was legalised in the UK last year - allows parents with rare genetic mutations to have healthy babies, by replacing a mother's faulty mitochondrial DNA with another woman's during the IVF process.
Seeing as mitochondrial DNA is only ever passed down by women, for most females with mitochondrial disease, this is the only way they'll be able to have healthy children.
But with a ban still in place in the US - one that experts are encouraging the government to overturn, we should add - the procedure is still controversial.
In this latest case, the new technique was used on Jordanian parents by a US-based team in Mexico. The boy's mother carries Leigh syndrome, a fatal disorder of the nervous system that's passed down through mitochondrial DNA.
Unlike regular DNA, which is housed in the nucleus of the cell, mitochondrial DNA lives in our mitochondria - which, as we all know from high school biology, is the 'powerhouse of the cell' - and is only ever passed down by females.
While the baby's mother is healthy, around a quarter of her mitochondria carried the faulty Leigh syndrome genes, and, after almost 20 years of trying for a baby, her only two children had died from the condition, leading her to reach out to John Zhang at the New Hope Fertility Centre in New York.
There are several ways to make 'three-parent' babies - the technique that's approved in the UK is called pronuclear transfer, and it involves fertilising both the mother's egg and a donor's egg with the father's sperm.
Before these two fertilised eggs begin dividing into an embryo, the researchers replace the donor egg's nucleus with the mother's nucleus - giving them a fertilised egg with a healthy donor mitochondria, and the mother's DNA in the nucleus.
But the parents of the five-month-old baby are Muslims, and had ethical issues with a fertilised cell being destroyed. So Zhang used a different approach called spindle nuclear transfer, which works in much the same way, but the nucleus transfer is done before the eggs are fertilised.
This was all carried out in Mexico where "there are no rules" about three-parent babies, Zhang told Hamzelou.
The technique created five embryos, but only one developed healthily, and was implanted into the mother. The baby is now five months old and seems to be developing normally, with no signs of Leigh syndrome.
Despite having no guidelines to follow, Zhang made sure the technique was carried out ethically - first of all, his team selected a male embryo, which can't pass down the donated mitochondrial DNA, so can't transfer any potential problems resulting from the technique.
They also avoiding destroying any embryos in the process. "It's as good as or better than what we'll do in the UK," said cardiac pharmacologist Sian Harding, who reviewed the ethics of the UK procedure.
But there's still concern from a lot of scientists that the procedure could lead to health problems for the child in the future.
Right now, less than 1 percent of the boy's mitochondria carry the Leigh mutation, which doesn't seem to be affecting his development at all. But Bert Smeets from Maastricht University in the Netherlands, who wasn't involved in the research, told New Scientist that the child needs to be monitored closely, and the technique should be more rigorously tested before we can deem it 'safe'.
"We need to wait for more births, and to carefully judge them," he said.
But even though this is the first baby born using the new 'three-parent' technique, there are already children out there who have DNA from three parents, due to a slightly different method used in IVF in the US in the late '90s.
As Michael Le Page reported for New Scientist earlier this year: "Up to 17 people in the US may already have been born with donor mitochondria, because of a technique one clinic used to boost the success of IVF between 1997 and 2002 - when the FDA stepped in to stop it."
Some of those babies went on to develop genetic disorders, which is why many scientists are still reluctant to use the new technique.
Only time will tell whether it can safely help women with mitochondrial disease to have children - but with the birth of the first healthy child, it seems inevitable that the technique is going to be used again, and we'll be watching the cases closely.
Zhang's report on his technique hasn't been peer-reviewed or published as yet, but the case study will be presented by his team at the American Society for Reproductive Medicine's Scientific Congress in Salt Lake City in October.