A new investigation into rust on the Moon implicates Earth as the culprit.
Oxygen leaking out of Earth is likely responsible for the transformation of iron to hematite (Fe2O3) at the lunar poles. Lab simulations find it's the only explanation for both the abundance of hematite and the distribution pattern, giving us a new window into the complex chemical exchange between our planet and our Moon.
"We conducted a series of oxygen and hydrogen irradiation experiments to simulate lunar surface irradiation processes," writes a team led by planetary scientist Xiandi Zeng of the Macau University of Science and Technology in China.
"For the first time, our experiments demonstrate both the formation and reduction of hematite minerals."
Related: Bizarre Discovery Reveals The Moon Is Rusting, Even Without Liquid Water And Oxygen

The shocking discovery of hematite on the Moon was made a few years ago. Hematite forms from the oxidation of iron, a process more commonly known as rusting. This mineral is found widely on Earth, but the Moon has no atmosphere, just a thin exosphere, and it contains no oxygen.
In addition, the Moon is constantly bombarded with a stream of hydrogen from the solar wind. Hydrogen is a reducing agent that 'donates' its electrons to the materials it interacts with. Oxidation occurs due to a loss of electrons – so even if all of the correct elements were present for oxidation to occur on the Moon, the solar wind should cancel it out.
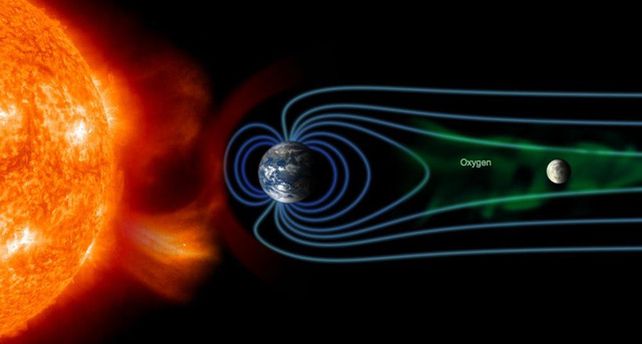
One possible explanation for the presence of hematite involved Earth. The solar wind pushing at Earth's magnetosphere causes the structure of said magnetosphere to trail out behind Earth in the direction opposite the Sun. This magnetotail also contains particles that leak out of Earth's atmosphere.
During the full Moon, terrestrial oxygen ions pelt our lunar satellite as it passes through Earth's magnetotail. Meanwhile, sitting in Earth's shadow means that 99 percent of the solar wind is blocked from reaching the Moon.
Theoretically, this means that the Moon spends approximately five days a month being bombarded with oxygen while experiencing reduced hydrogen bombardment – a potential recipe for hematite.
To test this in the lab, the researchers hurled oxygen ions at iron-rich minerals to mimic the effect of the Earth wind in the terrestrial magnetotail.
They selected pyroxene, olivine, ilmenite, troilite, and an iron meteorite as analog materials for the iron minerals that are known to exist on the Moon. They also experimented with magnetite (Fe3O4), confirming that the mineral is an intermediate step between metallic iron and hematite.
The results showed that oxygen ions are capable of oxidizing metallic iron, ilmenite, and troilite – but the effect was significantly stronger for metallic iron. Meanwhile, iron-bearing silicates such as pyroxene and olivine did not form hematite at all, suggesting that the process is selective.
"Our experimental results provide strong evidence that hematite can form on the lunar surface through oxygen ion irradiation. Earth wind, the primary source of energetic oxygen ions on the Moon, acts as the oxidant, driving the oxidation of various minerals, including metallic iron and iron-bearing oxides and sulfides abundant in lunar regolith," the researchers write in their paper.
"Although these iron-bearing minerals may occur as microparticles or small crystals in lunar regolith, they can undergo direct oxidation upon exposure to Earth's wind."
To determine whether the resumption of the solar wind can reverse this process rapidly enough to cancel it out, the researchers fired beams of hydrogen ions at hematite at different intensities. A high-energy beam mimicking Earth's wind was able to reverse the oxidation process, but a low-energy beam mimicking the solar wind did not.
This suggests that the solar wind is incapable of reversing the lunar iron rusting caused by a periodic influx of Earth oxygen. It also explains why hematite is concentrated near the lunar poles: Earth's magnetotail channels oxygen ions toward high latitudes while deflecting many hydrogen ions away.

The research may also solve another mystery about the Moon's hematite. It's often found near water, which scientists had considered a possible cause of the hematite.
Zeng and colleagues found water as a by-product in their reduction experiments: When they fired high-energy hydrogen at hematite, the oxygen detached from the iron and joined with the hydrogen. This suggests that the water near the lunar hematite is a by-product of hematite reduction.
There's a lot to explore there. Hematite on the Moon may even record the history of oxygen in Earth's atmosphere, which dates back to the Great Oxidation Event some 2.4 billion years ago.
"The formation of hematite (and potentially magnetite) via Earth wind irradiation underscores the material exchange between Earth and the Moon, which may have persisted for more than 4 billion years due to interactions between their coupled magnetospheres," the researchers write.
"These findings emphasize the need for further investigations into lunar regolith interactions with interstellar plasmas. More importantly, the recent successful landing of Chandrayaan-3 at 69°S, along with China's upcoming Chang'E-7 mission targeting the lunar south pole, presents promising opportunities to deepen our understanding of the intertwined history."
The research has been published in Geophysical Research Letters.
