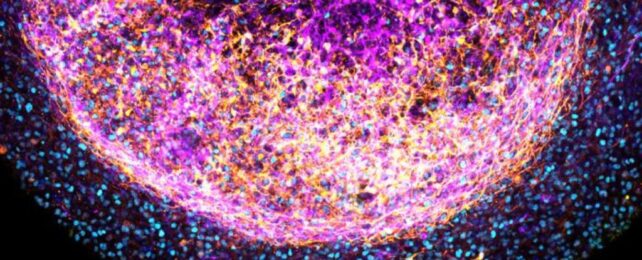
Scientists have found a way to skip some of the most challenging steps when building a miniature human brain model in the lab.
Instead of coaxing stem cells to proliferate into the millions and grow into different types of cells, like neurons, researchers in the Netherlands have managed to develop a brain organoid straight from fetal brain tissue.
The self-organizing structure that results is about the size of a grain of rice. While it is not a real organ (it has no thoughts, emotions, or consciousness), researchers hope it will prove to be a valuable model for the treatment of brain diseases and disorders, especially in children.
"With our study, we're making an important contribution to the organoid and brain research fields," says Hans Clevers, a pioneer in organoid research at the Hubrecht Institute and the Princess Máxima Center.
"Until now, we were able to derive organoids from most human organs, but not from the brain – it's really exciting that we've now been able to jump that hurdle as well."
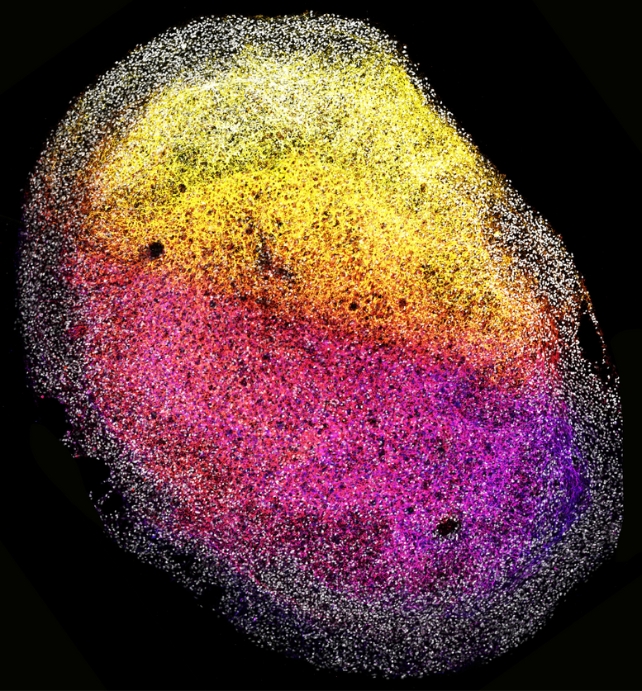
Fetal brain tissue is usually obtained from elective abortions, and its ethical allowances in scientific research vary considerably from nation to nation, with some countries outlawing its use entirely and others, like the Netherlands, allowing its use with strict limitations.
Given the low availability of fetal tissue, scientists have previously only grown human 'mini-brains' from stem cells.
But unlike cell-derived organoids, which spontaneously mature to an endpoint, tissue-derived organoids reflect a native, fixed developmental state that can be maintained for quite a while.
In the Netherlands, researchers at the Princess Máxima Center for pediatric oncology and the Hubrecht Institute worked closely with bioethicists to design their methodology.
Ultimately, the team was able to coax small fragments of fetal brain tissue to self-organize in a dish, creating a three-dimensional, layered structure with various cell types, including neurons and supporting cells, called radial glia.
The radial glia are an especially exciting development, as these are human-specific features that aren't replicated in rodent models.
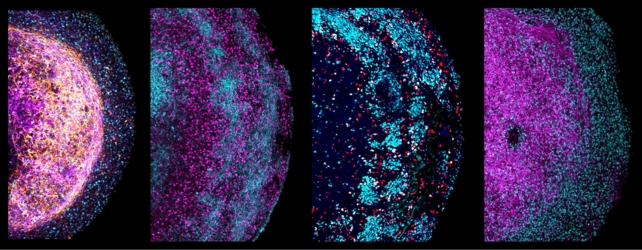
Even better, the brain organoid continued to respond to some of the same chemical signals as a living brain, and it stayed alive for more than six months.
By comparison, brain organoids made from stem cells can barely make it further than 80 days.
Clevers and his colleagues were even able to genetically manipulate their organoids to resemble cancerous tumors and test out drugs on them.
The team suspects their success can at least partly be attributed to the proteins that brain tissue can produce. These crucial proteins create scaffolding for brain cells so that they can self-organize into a three-dimensional structure.
"Our new, tissue-derived brain model allows us to gain a better understanding of how the developing brain regulates the identity of cells," says Benedetta Artegiani, research group leader at the Princess Máxima Center.
"It could also help understand how mistakes in that process can lead to neurodevelopmental diseases such as microcephaly, as well as other diseases that can stem from derailed development, including childhood brain cancer."
The study was published in Cell.