While many of the cells that make up our bodies are essentially squishy things, they do have some internal structures that need to (mostly) keep their shape. This includes the nucleus that safely stores our genetic instructions, tucked away in specific configurations.
Like all our cellular organs, the nucleus is anchored in place by a network of filaments called the cytoskeleton.
This cytoskeleton also plays an important role in how cells move, which determines crucial things such as how we develop, how organs function, and how cancer invades our bodies.
So far, cell movements have mostly been studied in a flat 2D environment. This is clearly quite different from the 3D worlds of our bodies, so a team of researchers from France took a closer peak at how cells navigate 3D obstacles, and captured some incredible footage.
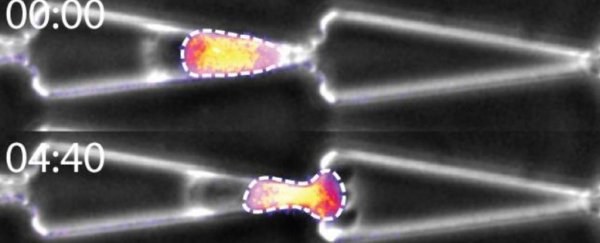
University of Strasbourg molecular biologist Emilie Le Maout and colleagues set up an obstacle course of tunnels. Some were open, and others had constrictions, with some bottlenecks smaller than a cell's nucleus.
As their video shows below, the fibroblast cells they tested - cells that make up connective tissue important for wound healing and collagen formation - can actually distort themselves to squeeze through.
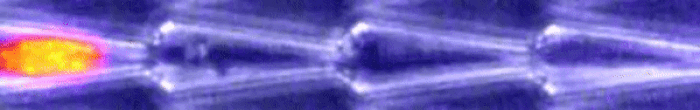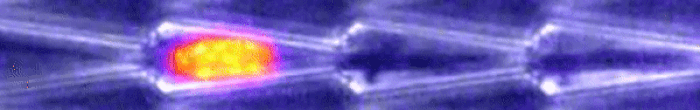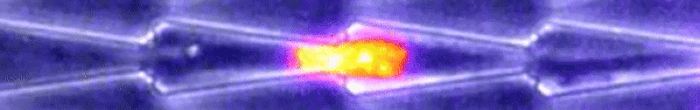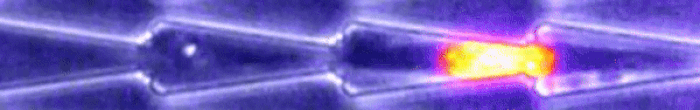 (Le Maout et al, Biophysical Journal, 2020)
(Le Maout et al, Biophysical Journal, 2020)
The team also discovered that when the gap is too small for the nucleus to fit, the cells pause. Some cells then seem to anchor themselves and pull until their nucleus squashes enough to get through as well.
Another video caught how keratin gathers at the trailing end of the nucleus during this squishing process. Keratin is a component in one of three filament networks that makes up the cytoskeleton. It plays a role in rapidly building and disassembling these scaffolds.
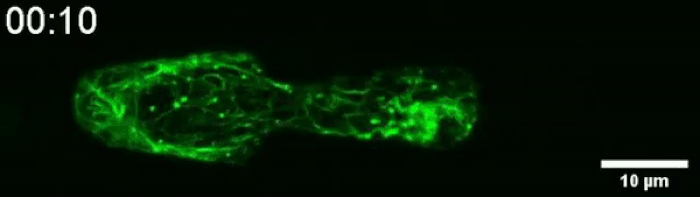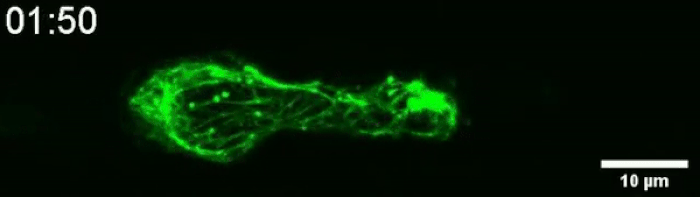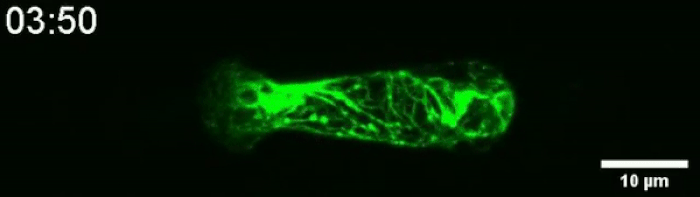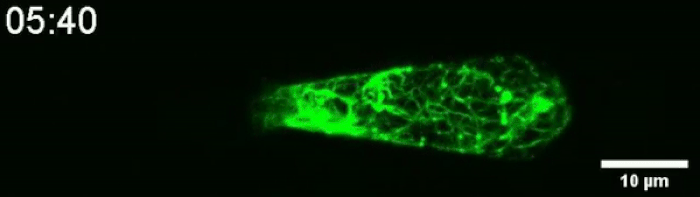 (Le Maout et al, Biophysical Journal, 2020)
(Le Maout et al, Biophysical Journal, 2020)
This keratin rearrangement may be what allows the cell to distort, so Le Maout and team tested this theory with mutant oral squamous epithelial cells - the cells that line your mouth.
The mutation produces deformed keratin proteins that are linked to cancers found in this tissue type. The mutant cells couldn't get past the bottlenecks, suggesting keratin is critical to the squishing process, perhaps of the nucleus.
"Because initial arrest in the capillary is critical for tumour cells to metastasize to secondary sites in distant organs, blockage by mutant keratin may provide advantages for tumour seeding, survival, and proliferation," said University of Strasbourg cell biophysicist Daniel Riveline.
"Future studies could take this channel strategy to identify signalling networks that are modified in the context of cancer."
Previous research has shown there is a limit to how far the nucleus can be distorted. It bursts if squashed too far, releasing the precious DNA into the cell's cytoplasm, which contains DNA-destroying enzymes that protect against viruses.
A tear in the nuclear membrane "is the worst thing that can happen to a cell and have the cell survive," cell biologist Matthieu Piel told Science in 2016. The cells have mechanisms to rapidly repair this damage, but some of it can lead to cell death or cancer.
Le Maout and team also found the cells they tested would move in directions of chemical gradients when these were present, otherwise venturing towards areas of broken symmetry. Lacking these breaks in uniformity, the cells would continue in the direction they're facing when they enter a new tube.
Understanding more about the rules and limits of cellular movements could reveal new ways to repair them when cells go wrong - a subtle, yet vital step for our understanding of cancer.
This research was published in the Biophysical Journal.