The Pauli exclusion principle is a rule in quantum mechanics that explains why only a limited number of electrons can occupy any one of an atom's orbitals.
Predicted by Austrian theoretical physicist Wolfgang Pauli in the 1920s, the exclusion principle underpins basic chemistry, and helps explain why massive objects like neutron stars and white dwarfs resist gravity crushing them into infinitely small black holes.
We're all intimately familiar with its day-to-day effects, in the form of atoms pushing and pulling into structures made of molecules thanks to the interactions of their electrons.
But to understand why this separation occurs we need to think of electrons in a completely non-intuitive way, as both virtually indistinguishable spheres of matter and as smeared-out waves of possibility.
Why can't electrons sit in the same seat?
Take an ordinary atom and zoom in close on one of its electrons.
This tiny point of matter might be described by just a handful of characteristics, such as charge, mass, angular momentum, and a location around the nucleus.
We can also describe similar characteristics using simple values that depend on a particular energy system. These values are the electron's four 'quantum' numbers.
Pauli's exclusion principle states two or more bound electrons can't have the same four quantum numbers when in the same system. Particles with the same energy, magnetic quantum number, intrinsic angular momentum, and orbital angular momentum simply cannot sit in the same seat around an atom's center stage.
The reason for this is more a feature of mathematics than anything, so it can be a little hard to picture.
To appreciate it, we need to stop thinking of electrons as tiny solid objects (like the picture below on the left) and remember they are more like flickering 'ghosts' that haven't really worked out where to appear yet (like the four different examples of 'zones of possibility' on the right.
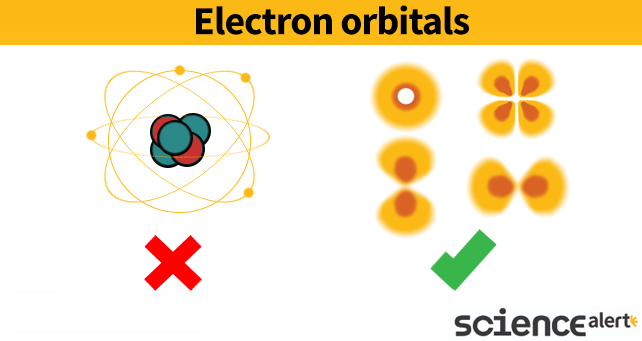
All of those quantum characteristics can be placed into an equation that describes how this ghost's fate might evolve over time. Because it's not a certainty, this equation can be mapped out as a wave describing a rise and fall of likelihood.
Tinkering with the mathematics of this wave reveals a few facts. One is that any two particles sitting at the same level of energy around a nucleus – with otherwise identical characteristics – would behave nothing like electrons. The equation would now look different, more in line with what a photon of light might look like.
By changing a quantum number in one of the electrons, such as by giving it a spin that goes in the opposite direction, we can make the wave look electron-like again.
Putting it another way, a fundamental part of an electron's wave-like behavior forbids them from overlapping perfectly with one another. It's a principle of identity that applies not just to electrons but to all subatomic particles that belong to the group of fermions.
The rule of quantum numbers helps us better understand the physics behind chemistry. Just two electrons can sit happily together in an atom's lowest orbital, for example, each with one of two kinds of spin number.
From there, things get more complicated. Further away, there are more options for quantum numbers to vary, allowing for specific numbers of electrons to occupy the space.
Chemists know these electron numbers well, as they determine how different elements can overlap and form bonds.
Astronomers also note the consequences of this exclusion principle – it takes an incredible amount of energy to force electrons into spaces they don't typically move in, such as the inside of protons, which helps explain the workings of exotic objects like neutron stars. Push hard enough, and they may even lose all identity, mushing together into the space of a black hole's core.
For the rest of us, we can just be comfortable that such exclusive rules of chemistry keep us from falling through the ground in a blur of random ghostly emptiness.
All Explainers are determined by fact checkers to be correct and relevant at the time of publishing. Text and images may be altered, removed, or added to as an editorial decision to keep information current.
