Specially engineered 'young' immune cells could help to reverse the effects of aging and the damage to brain cells caused by diseases such as Alzheimer's, according to a new study in mice.
In their natural state, these immune cells are known as mononuclear phagocytes, and they flow around the body, cleaning up waste.
As we get older, however, these immune-cell cleaners get a bit sloppy, clearing away less cellular debris and triggering more inflammation than before. Inflammation and protein aggregation are features of many age-related diseases, including Alzheimer's.
So researchers from Cedars-Sinai Medical Center in the US produced replacement squads of mononuclear phagocytes, using human induced pluripotent stem cells – starter cells that can be reprogrammed into many different cell types.
Related: Alzheimer's And Cancer May Soon Be Treated With Sounds We Can't Hear
"Previous studies have shown that transfusions of blood or plasma from young mice improved cognitive decline in older mice, but that is difficult to translate into a therapy," says biomedical scientist Clive Svendsen.
"Our approach was to use young immune cells that we can manufacture in the lab – and we found that they have beneficial effects in both aging mice and mouse models of Alzheimer's disease."
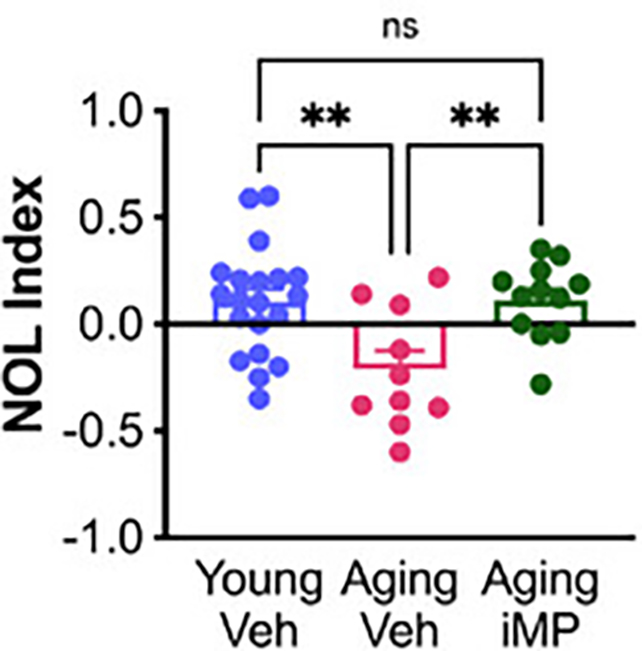
The treated mice did better on memory tests, the researchers found, while they also developed healthier microglia in their brains.
Microglia are the central nervous system's mononuclear phagocytes. They help reduce damage and waste in the brain, but become depleted in older brains and the those of people with Alzheimer's.
Another observed benefit was an increase in the number of mossy cells that help the brain's memory control center, the hippocampus, function properly. Like microglia, these mossy cells can be hit by old age and Alzheimer's.
"The numbers of mossy cells decline with aging and Alzheimer's disease," explains neuroscientist and lead author Alexandra Moser.
"We did not see that decline in mice receiving young mononuclear phagocytes, and we believe this may be responsible for some of the memory improvements that we observed."
The researchers originally hypothesized that mononuclear phagocytes could be part of the reason that blood plasma and bone marrow transfusions have the rejuvenating effects they do, and that idea was supported by the results here.
However, the engineered immune cells that Moser and colleagues injected into mice didn't appear to reach the animals' brains, so the researchers figured the observed effects must be down to something produced by fitter, stronger mononuclear phagocytes circulating in the body.
The 'young' cells may have released anti-aging proteins or tiny parcels involved in cell-cell communication, called extracellular vesicles, that made their way to the brain, reducing inflammation and boosting the immune system.
Related: New Alzheimer's Treatment Clears Plaques From Brains of Mice Within Hours
It's important to note that most of the benefits observed here were in the older mice, not the mice engineered to display signs of Alzheimer's disease. There was also plenty of Alzheimer's-related brain damage, including amyloid-beta protein buildup, that wasn't repaired.
And there's no guarantee we'll see the same effects in human brains either.
Despite those limitations, the early signs of this approach are promising. If derived from a patient's own cells, engineered mononuclear phagocytes could alleviate some of the drawbacks of blood plasma transfusions and bone marrow transplants, so there's plenty of potential for future research.
"These findings show that short-term treatment improved cognition and brain health, making them a promising candidate to address age- and Alzheimer's disease-related cognitive decline," says Cedars-Sinai Medical Center neuropathologist Jeffrey Golden, who wasn't directly involved in the study.
The research has been published in Advanced Science.