For most of us, diagrams of atoms as tiny solar systems serve as our introduction to the world of particle physics. Unfortunately, these iconic images get more wrong than they get right.
Electrons aren't like tiny planets orbiting a lumpy sun, for one thing. They're probably not even tiny spheres, but rather pin-pricks in space without width, height, or depth.
And as an object without a width, it has nothing that can rotate.
So what are physicists referring to when they describe a particle's 'spin'?
Why do we think electrons spin?
In the early days of quantum mechanics, a number of physicists pondered the idea of whether particles like electrons did in fact spin around. While the idea didn't fit neatly with existing theories on electron behavior, there were a handful of observations in experiments and niggling gaps in theory that said otherwise.
One is the fact an electron's path curves as it encounters a magnetic field, as if it is a tiny magnet itself. This itself isn't a shocking thing – moving charges create magnetic fields, after all.
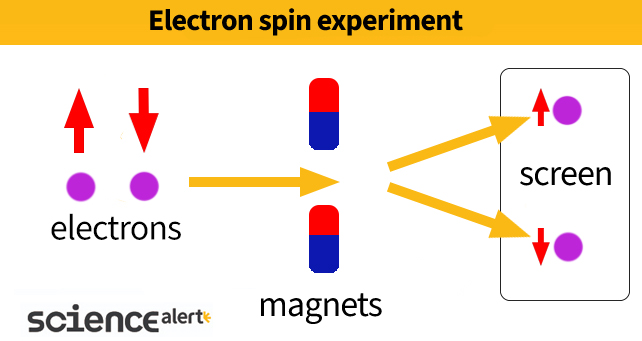
But when two German scientists named Otto Stern and Walther Gerlach measured this field in electrons orbiting the nucleus of silver atoms in the early 1920s, they found the numbers didn't add up. The electrons had to also be moving on the spot for it to make sense – they had to be spinning.
Strangely, the results implied that such spinning created small magnetic fields of very specific orientations, strictly pointed straight up or straight down with respect to an external magnetic field. They were never randomly tilted one way or another.
Meanwhile a theorist named Wolfgang Pauli was working on a principle that helped explain why some particles (such as electrons and particles in the atom's nucleus) couldn't sit on top of one another while occupying the same space, while others (like photons) could.
His ' exclusion principle' required a set of four quantum numbers. One described the energy of a particle. Two others had to do with angular momentum. But the fourth didn't seem to relate to anything obvious.
A young Dutch-born American scientist named Samuel Goudsmit would soon provide the answer. Applying new interpretations of formulae on spectral lines in magnetic fields called doublets, he'd unwittingly uncovered evidence of a movement in electrons that appeared uncannily like spin. Not that he saw it at first - it took many long conversations with another young Dutch-American physicist named George Uhlenbeck to make it clear.
"But don't you see what this implies? It means that there is a fourth degree of freedom for the electron," Uhlenbeck famously responded.
"It means that the electron has a spin, that it rotates."
Though they weren't the first to consider the concept, the conversation, along with the results of such theories and experiments, generated a clear-cut case for electrons - among other fundamental particles - rotating.
But nothing can be so simple down the rabbit hole of quantum mechanics.
Why can't electrons rotate?
Even as the term spin was being used to describe the weird magnetism of electrons and the strange properties of light, there were a few problems.
If we were to assume electrons were truly tiny spheres, the speed at which they would have to rotate to match experimental results would mean their surface would have to whip around ten times faster than the speed of light.
Of course it's now thought that electrons don't have a surface. Knowing fundamental particles are points in a field doesn't make it any more intuitive, though. How does a zero-dimensional dot turn around in the first place?
More confusing still are the experiments Stern and Gerlach conducted, which only ever point in two absolute directions. The distinct 'totally up' versus 'totally down' orientations of a particle's tiny magnet aren't easily explained by a whirling 3D object, which can tilt a little this way, a little that way, or speed up and slow down.
In other words, the kind of spin taking place in electrons doesn't have an equivalent in our world of tops, basketballs, and planets.
It might have similar consequences, making particles curve much like they have intrinsic angular momentum and turning them into a weird kind of bar magnet. But whatever 'spin' happens to be, it's a fundamental property meshed into the very fabric of our Universe.
All Explainers are determined by fact checkers to be correct and relevant at the time of publishing. Text and images may be altered, removed, or added to as an editorial decision to keep information current.
