Robotic skeletons designed to look and move like real humans are being investigated for growing tendons strong enough to actually transplant.
If the proof-of-concept can be perfected with further research, tissue grown on humanoid robots could one day be grafted onto a real person, fixing tears in their tendons.
Today, surgeons can try their hands at repairing tendons, often transplanting a graft from another tendon in the body, but the outcomes are mixed. For more than two decades, scientists have been looking into alternative strategies, like artificially engineering tendons for transplantation.
Growing new tendons from an individual's cells outside of the body, however, is tricky business. It's currently done in little bioreactor chambers, which simulate the conditions of a joint.
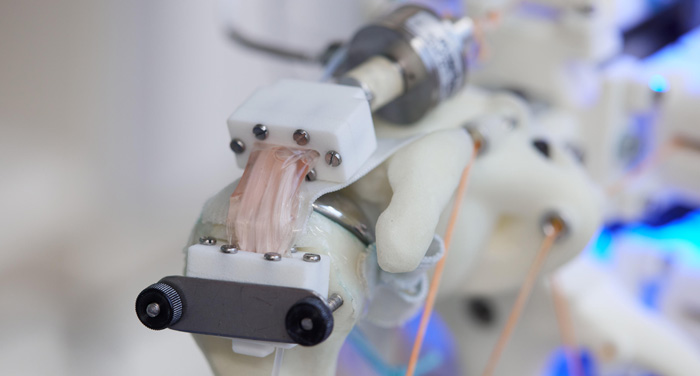 Close up of the soft transparent chamber hosting a cell-material construct. (Fisher Studios)
Close up of the soft transparent chamber hosting a cell-material construct. (Fisher Studios)
Existing evidence suggests dynamic movement like stretching and flexing is key in tendon development, but even the most cutting edge bioreactors fall short in mimicking the range and magnitude of movements expected of a tendon. The result is tissue that may not live up to the task required of it.
Robots could help solve that problem by essentially 'breaking in' the tendons for us.
So far, the concept has only been proved using a simplified, robotic shoulder joint. But when human cells derived from a shoulder tendon were grown in a flexible bioreactor chamber – one that could bend and extend with a robot's arm – they proliferated faster than those nurtured in a static environment.
The cells also expressed different genes.
Mechanical stress – like tension, compression, and torsion – occurs when human tendons grow naturally, so it makes sense that similar movements could help engineered versions grow, too.
Tendons are what connect human muscles to our bones, and this means they must be both strong and flexible.
If tendons are deprived of tensile stress, they rapidly deteriorate. In fact, the very composition of the tissue begins to unravel.
The tendons of the human shoulder are especially busy. This joint has the greatest range of movement in the entire human body, which means tendons are hard at work keeping the ball and socket in place.
The supraspinatus muscle and tendon is the line of tissues that connects the shoulder blade to the arm's humerus. It's mostly involved in helping the arm flap from a person's side upwards.
When researchers grew cells from the supraspinatus tendon in a flexible bioreactor and then attached that bioreactor to a robotic shoulder based on human anatomy, they were able to mechanically manipulate the cells to move.

The repeated motions of flapping, known as arm abduction and adduction, seemed to give the developing tissue some flexibility and strength, reducing stiffness.
After 14 days, the force of the robotic movements clearly influenced the growth of the human cells.
The proof-of-concept suggests that flexible bioreactors, when attached to humanoid robots, are more realistic platforms on which to engineer tendons.
But there's still a lot of testing that needs to be done. Researchers want to investigate what bioreactor materials are best to use, what cell types respond to pushes and pulls best, and which robotic movements are most useful to grow human tissue.
"Possible long-term benefits from a humanoid bioreactor-based strategy include the production of functional tissue grafts for patients, the creation of an improved in vitro culture model for preclinical work and the opportunity to support the development of advanced robotic systems," they write.
The study was published in Communications Engineering.
