More than 80 years after it was first predicted, physicists have created metallic hydrogen - a mysterious form of hydrogen that could be capable of superconducting electricity without resistance at room temperature.
Scientists have long suspected that hydrogen could exist as a metal in certain parts of the Universe, but this is the first time metallic hydrogen has ever been created on Earth, and the material is even stranger and more fascinating than scientists imagined.
"This is the holy grail of high-pressure physics," says lead researcher Isaac F. Silvera from Harvard University. "It's the first-ever sample of metallic hydrogen on Earth, so when you're looking at it, you're looking at something that's never existed before."
The periodic table can be broadly be split up into two categories - metals and non-metals. Among many other properties, metals are lustrous (shiny), good conductors, and usually solid at room temperature, while non-metals have a dull appearance, and are poor conductors.
As most of us learnt at high school, hydrogen - the first element on the periodic table - is a non-metal.
But back in 1935, researchers predicted that under certain conditions, this common and oft-studied element could have its atoms bind together so tightly, the material wouldn't just take on metallic properties, it could actually become a metal.
But those conditions aren't easy to achieve - they involved achieving incredibly high pressures at extremely low temperatures, which is why for more than 80 years, and despite numerous attempts, no one had been able to prove it was possible, until now.
"The most exciting part is we pressurised hydrogen gas to sufficiently high pressures and we saw it convert into a metal," Silvera told ScienceAlert.
Silvera has been trying to create metallic hydrogen for 45 years.
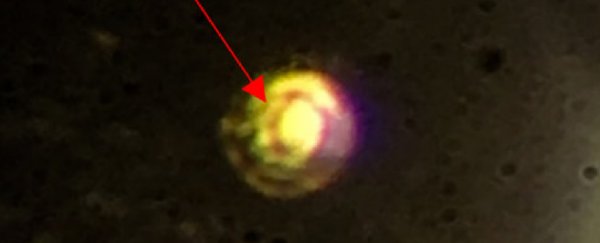
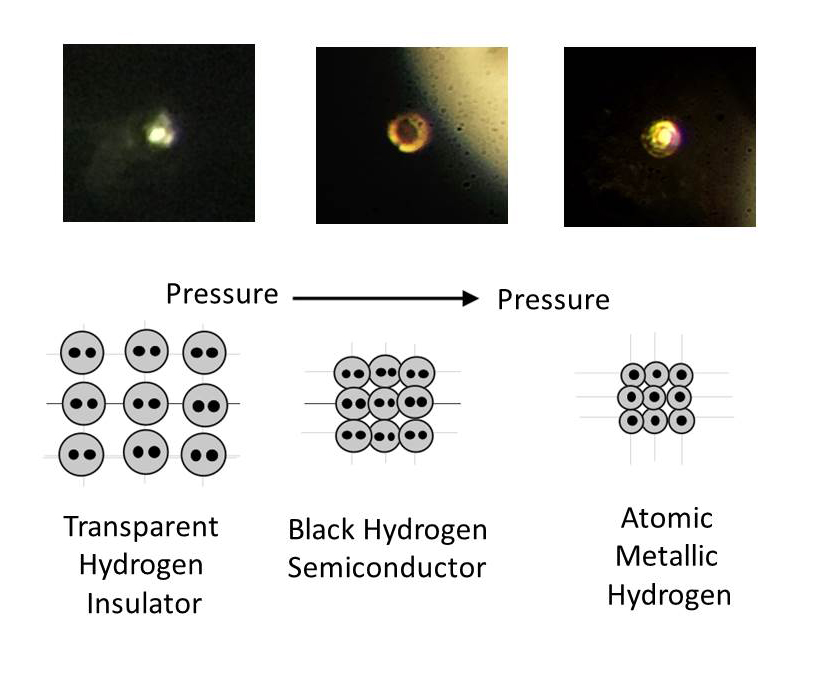
"The hydrogen went from being transparent, to non-transparent and black, and suddenly it became lustrous," he explained. "We could actually see it become a metal."
You can see this material, for the first time ever on planet Earth, below:
 Silvera et al
Silvera et al
This isn't just exciting as a proof-of-concept in the physics world - although it's definitely that. Metallic hydrogen has been the source of so much speculation over the years because it's predicted to have some incredible properties.
Most importantly, physicists think that metallic hydrogen could be a room-temperature superconductor, which would mean the material could conduct electricity with zero resistance - and without having to be cooled to crazy temperatures first.
We know of many superconducting materials already - we use them to create the powerful magnetic fields in our MRI machines and in maglev trains - but they're only capable of achieving superconductivity at temperatures below –269 degrees Celsius (–452.2 degrees Fahrenheit), which makes them expensive and non-practical for many purposes.
If scientists could achieve that same superconductivity at room temperature, it would be huge, because it means we could create things like power lines that don't lose any electricity between the power plant and your home. Right now, the grid loses as much as 15 percent of its energy as heat, due to resistance.
The material could also be the most powerful rocket propellant ever discovered, with incredible energy stored up in its bonds capable of blasting us to distant worlds.
To be clear, the metallic hydrogen that Silvera and his team have created is only around 1 to 1.5 microns thick, and 10 microns in diameter, so it's tiny.
And until peer-review had confirmed that their sample was the real deal, they were hesitant to perform too many tests on it, so we have no evidence so far to suggest that the material is a superconductor. That's something that will be investigated in the months to come.
But for now, we know the sample is real, and it's been stable in Silvera's lab since October.
Researchers have claimed that they've made the early stages of metallic hydrogen in the past - and even claimed evidence of metallic hydrogen itself. But these reports have never been verified. This latest claim will now have its chance to have holes poked in it by critics, but so far the sample has withstood all relevant metallic testing.
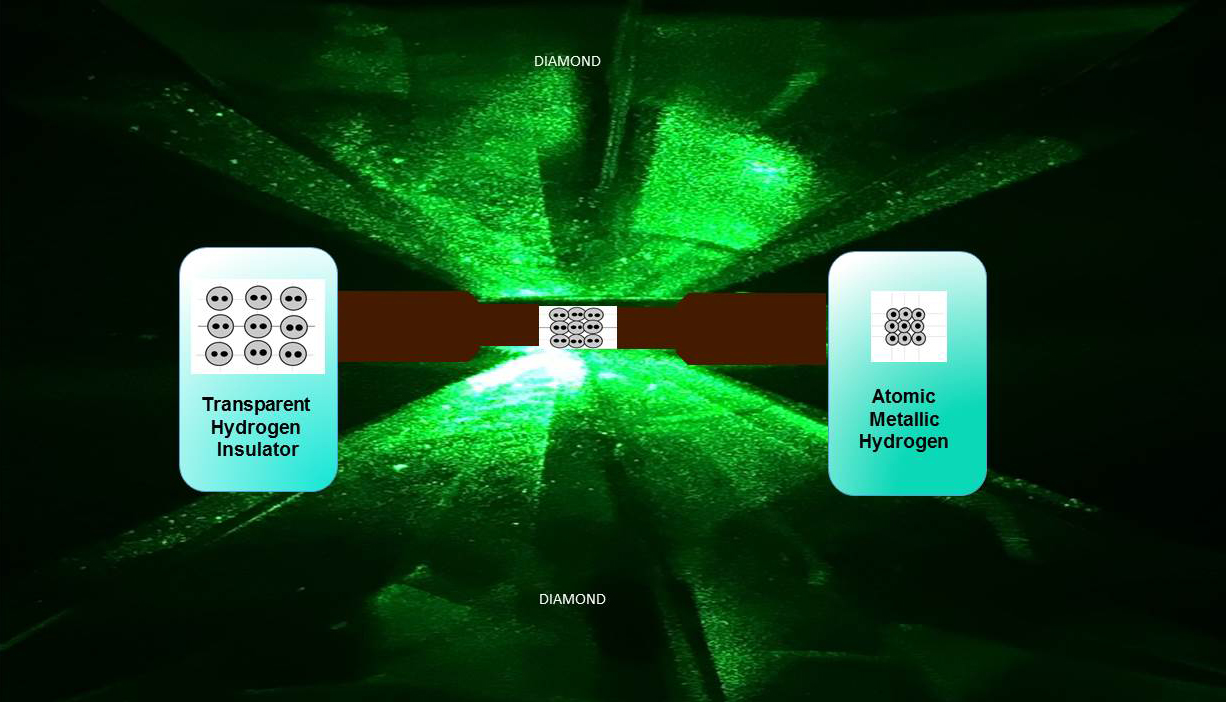
To create the sample, the team trapped hydrogen gas inside a tiny diamond casket, chilled it to 5.5 Kelvin (–267.65 degrees Celsius and –449.77 degrees Fahrenheit) and put it under incredibly high pressure.
 Silver et al
Silver et al
And when we say high pressure, we mean high pressure. Back in 1935, it was predicted that metallic hydrogen would emerge at 25 gigapascals (GPa) of pressure. But Silvera and his team finally achieved it at pressures between 465 and 495 GPa - nearly 20 times higher than initially predicted.
For perspective, 1 GPa equals 1 million kilopascals (KPa), and the average pressure at sea level on Earth is 101.325 KPa.
The team quickly saw its appearance change, but to verify that what they'd created was metallic hydrogen, they used spectroscopic measurements, including measuring its reflectivity, and showed that what was originally a standard hydrogen gas (H2) had transformed into an atomic metal.
You can see the different atomic structure below:
 Silver et al
Silver et al
Now that we know metallic hydrogen exists, there are many questions left to be answered. The biggest of these is whether or not metallic hydrogen is a liquid or a solid - as researchers have predicted it could be both.
So far, Silvera and his team believe that what they've created is a solid, but they'll be performing more detailed analyses of the material now that it's been verified (something they weren't willing to risk before in case they inadvertently destroyed the fragile sample).
They'll also be hooking the metal up with current to test whether it really is a superconductor at room temperature - something that's possible whether it's a liquid or solid.
"It's going to be challenging but we're going to try," says Silvera.
Oddly enough, Silvera says that it's also likely that metallic hydrogen could be metastable - which means that even if you release the pressure it will remain metallic.
A common example of a metastable material is diamond, which is a metastable form of carbon. To make diamond, you put graphite under incredible pressure and heat - something that happens naturally deep below Earth's surface.
But even when you dig diamonds up out of the ground, they remain diamonds.
The same might be true with metallic hydrogen, and this is something that will also be tested once all other analysis has been performed on the sample, just in case the predictions are wrong, and the material disperses back into a gas when the pressure is lifted.
"We're going to work on this sample for a while, and then we'll release the pressure and see if the sample persists as metallic hydrogen," says Silvera. "And then we're going to load another sample."
There are exciting times ahead, and many more discoveries to be made. But today we just proved that the most common of all the Universe's elements can exist in an entirely new form, and that's cause enough for us to celebrate.
The research has been published in Science.