Water frozen in the darkness of space doesn't appear to behave the way we thought.
A new research effort using computer simulations and experiments to explore the most common form water takes in the Universe has found that it is not as structureless as scientists had thought. Rather, repeating patterns – otherwise known as crystals – just a few nanometers across are likely embedded in an otherwise frozen jumble of molecules.
Since scientists had thought space too cold for ice crystals to have the energy to form, this discovery comes as a big surprise.
"We now have a good idea of what the most common form of ice in the Universe looks like at an atomic level," says physicist Michael Benedict Davies of University College London and the University of Cambridge in the UK.
"This is important as ice is involved in many cosmological processes, for instance in how planets form, how galaxies evolve, and how matter moves around the Universe."
Related: Scientists Discover a Weird New Form of Ice That May Change How We Think About Water
There's no getting around it: water is, for all its necessity to life on Earth, pretty strange stuff. It doesn't behave like other liquids, and scientists have identified at least 20 or so distinct phases that it takes on under various frozen conditions.
Broadly, water ice falls into two distinct categories. Crystalline ice is what we have here on Earth, where the atoms are arranged in a neat crystalline lattice. In space, scientists thought, ice ought to be amorphous: a frozen agglomeration of atoms all clumped in higgledy-piggledy and any-which-way.
However, some analyses suggest that at least some forms of amorphous ice may be partially crystalline, so Davies and colleagues conducted computer simulations and experiments to investigate.
Space frostwork on @Space_Station window#ISS pic.twitter.com/dOXimzkOmn
— Serg.Korsakov (@SergKorsakov) May 23, 2022
The simulations involved freezing virtual containers of water molecules down to temperatures around -120 degrees Celsius (-184 degrees Fahrenheit) at different rates. Different freezing rates produce solids in varying proportions of amorphous and crystalline ice, wherein some of the ice is arranged in neat grids, and some is not.
Previous studies have hurled X-rays at amorphous ice to discern its structure in the way beams bounce off the interior of the material. The team's results showed that the proportion that best matches these experiments was around 20 percent crystalline and 80 percent amorphous.
In their experiments, the researchers then created amorphous ice in different ways. In space, water has no liquid form, instead freezing directly from vapor onto surfaces, such as rocks. To mimic this process, the researchers deposited water vapor onto a cold surface to freeze.
They also crushed ice at extremely cold temperatures to create a higher-density form of amorphous ice. Then, the researchers warmed each ice to the point where it would have enough energy to form crystals.
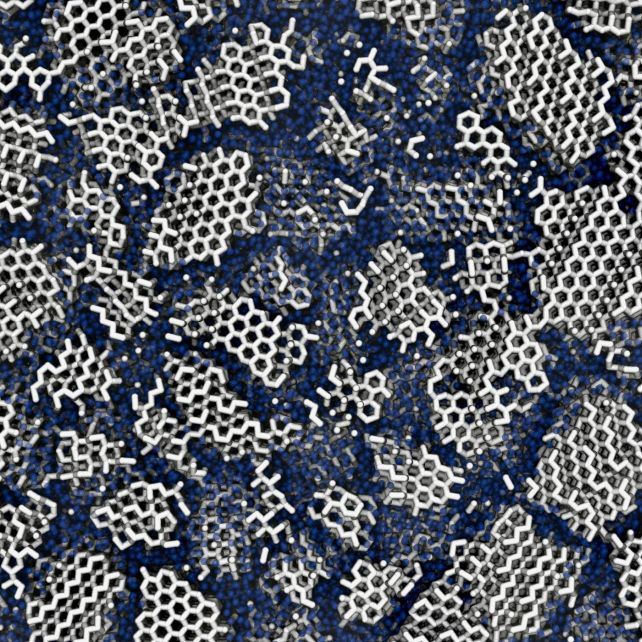
It's known that ice can 'remember' its previous structure; specifically, the order in which its hydrogen atoms have been arranged while in a crystalline state. That order can be retained even as conditions change.
When the researchers warmed up both their ices, they found differences in the structure that indicate that amorphous ice contains crystals: if it didn't, it would remain fully amorphous.
Although these experiments were conducted here on Earth, the findings constitute evidence that ice in space may indeed contain tiny regions of crystallization, the researchers say. This has implications for understanding not just water in space, but amorphous materials in general.
"Ice on Earth is a cosmological curiosity due to our warm temperatures. You can see its ordered nature in the symmetry of a snowflake. Ice in the rest of the Universe has long been considered a snapshot of liquid water – that is, a disordered arrangement fixed in place. Our findings show this is not entirely true," says physical chemist Christoph Salzmann of University College London.
"Our results also raise questions about amorphous materials in general. These materials have important uses in much advanced technology. For instance, glass fibers that transport data long distances need to be amorphous, or disordered, for their function. If they do contain tiny crystals and we can remove them, this will improve their performance."
The research has been published in Physical Review B.
