To study the inner workings of an atom's nucleus, scientists have traditionally relied on sophisticated particle colliders to blast nuclei apart with electrons.
These colliders often require large facilities, some spanning kilometers, that can accelerate electrons to breakneck speeds in search of secrets within nuclei.
In a new study, researchers suggest a simpler, much smaller-scale alternative. They gleaned data from inside nuclei without all the hullabaloo, instead enlisting an atom's own electrons as "messengers" within a diatomic molecule.
Related: Huge 56-Mile Particle Smasher Is Possible, Says CERN Report
They did this by pairing a radium atom with a fluoride atom, forming a molecule of radium monofluoride. Capitalizing on properties of the intramolecular environment, they created a kind of microscopic collider in which the radium atom's electrons briefly infiltrated its nucleus.
This let the researchers precisely monitor energies of electrons within the molecule, which revealed a subtle energy shift. Electrons were evidently making brief forays into the radium nucleus and interacting with its contents.
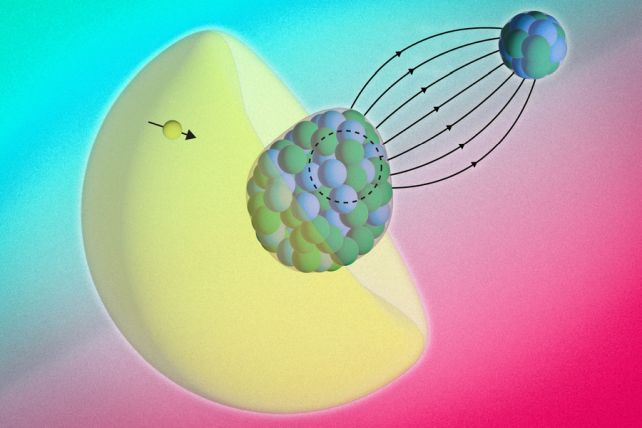
That could represent a novel way to measure the magnetic distribution of a nucleus, or how its arrangement of protons and neutrons influences its magnetic properties.
The new study is an early step, the researchers note, but they plan to use this technique to shed a new light on the radium nucleus. This kind of insight could help solve key mysteries in physics, such as why the Universe seems to contain far more matter than antimatter.
"Our results lay the groundwork for subsequent studies aiming to measure violations of fundamental symmetries at the nuclear level," says MIT physicist study co-author Ronald Fernando Garcia Ruiz. "This could provide answers to some of the most pressing questions in modern physics."
Current models suggest the baby Universe must have held roughly equal amounts of matter and antimatter, yet the latter is suspiciously rare. Instead, we find mostly matter in the Universe today, an apparent violation of the expected symmetry between the two.

Scientists suspect answers lurk within certain atomic nuclei, whose innards could contain clues about the scarcity of their antimatter counterparts.
Radium is a prime candidate, the researchers explain, due to the pear-like shape of its nucleus. Most atomic nuclei are spherical; radium's asymmetrical architecture might boost the observability of fundamental symmetry violations.
"The radium nucleus is predicted to be an amplifier of this symmetry breaking, because its nucleus is asymmetric in charge and mass, which is quite unusual," Garcia Ruiz says.
It's still a tough nut to crack, though.
"Radium is naturally radioactive, with a short lifetime and we can currently only produce radium monofluoride molecules in tiny quantities," says lead author and physicist Shane Wilkins, a former MIT postdoc now at Michigan State University. "We therefore need incredibly sensitive techniques to be able to measure them."
The key is to embed a radium atom in a molecule, which contains and intensifies the activities of its electrons, explains co-author Silviu-Marian Udrescu, a physicist at Johns Hopkins University who contributed to the study as a graduate student at MIT.
"When you put this radioactive atom inside of a molecule, the internal electric field that its electrons experience is orders of magnitude larger compared to the fields we can produce and apply in a lab," Udrescu says. "In a way, the molecule acts like a giant particle collider and gives us a better chance to probe the radium's nucleus."
Within radium monofluoride, the radium atom's electrons were constrained in a way that boosted their odds of entering the nucleus. Researchers confined and cooled the molecules, then used lasers to measure the energies of electrons inside them.
Tiny but significant shifts in the data hinted at ventures inside the nucleus.
"There are many experiments measuring interactions between nuclei and electrons outside the nucleus, and we know what those interactions look like," Wilkins says.
"When we went to measure these electron energies very precisely, it didn't quite add up to what we expected assuming they interacted only outside of the nucleus," he adds. "That told us the difference must be due to electron interactions inside the nucleus."
This discovery could revolutionize our ability to study atomic nuclei, the researchers report. Although we know how stubbornly elusive subatomic particles can be; they don't give up their secrets easily.
"We now have proof that we can sample inside the nucleus. It's like being able to measure a battery's electric field. People can measure its field outside, but to measure inside the battery is far more challenging. And that's what we can do now," Garcia Ruiz says.
"Radium-containing molecules are predicted to be exceptionally sensitive systems in which to search for violations of the fundamental symmetries of nature," he adds. "We now have a way to carry out that search."
The study was published in Science.