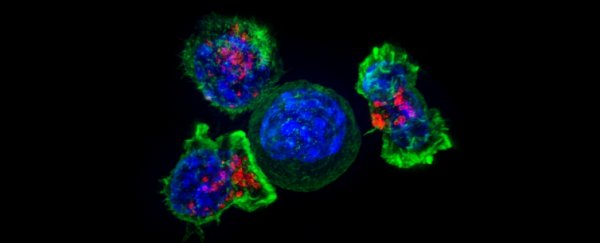
One of the challenges in treating cancer is stopping it from metastasizing, and a new study reveals one of the fundamental mechanisms through which this happens. Now we know about this mechanism, perhaps we can stop it.
Key to this newly discovered process is GRP78, and it's what's known as a chaperone protein. It's a type of protein that lends a hand in the folding or unfolding of larger proteins, basically building them up (or tearing them down), which then affects whether they're biologically active and functional.
A team led by the Keck School of Medicine at the University of Southern California (USC) in the US found that cancer cells can hijack GRP78, using the protein to spread further in the body and resist treatment.
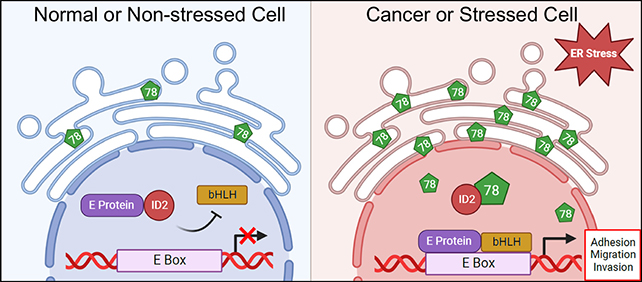
This appears to happen because the protein migrates when under stress. GRP78 is usually found in the endoplasmic reticulum part of a cell, but this research shows it moving to the nucleus and changing the cell behavior.
"Seeing GRP78 in the nucleus controlling gene expression is a total surprise," says Amy Lee, a biochemist and molecular biologist at USC.
"When it comes to the basic mechanisms of cancer cells, this is something novel that, to my knowledge, no one has observed before."
The discovery came about by analyzing how GRP78 regulates the gene EGFR, previously linked with cancer. Advanced 2D and 3D imaging techniques – including confocal microscopy, where separate beams of light are used to increase resolutions, were used to confirm the migration of GRP78.
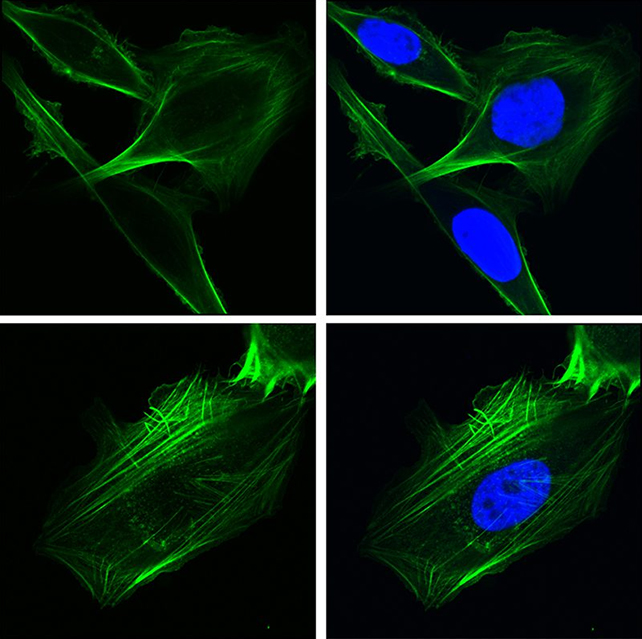
Further methods, including RNA sequencing (taking a snapshot of cell activity), were then used to get a closer look at what the GRP78 protein was actually doing. It turns out that the key genes it was regulating were involved in cell migration and invasion.
Another important discovery made by the researchers was how GRP78 binds or interacts with another cellular protein, ID2. It seems that GRP78 stops ID2 from doing its regular job, which is limiting the activity of genes involved in cell migration, including EGFR.
While this might all seem very technical, once scientists understand how proteins like GRP78 operate regarding cancer metastasis, they can start thinking about ways to control them. By stopping GRP78 from moving around or blocking ID2, for example, we might be able to prevent cancer cells from spreading.
Getting to that stage will require a lot more research, but discoveries are being made on a regular basis when it comes to cancer growth – whether that's how they get into the blood or spread to the bones.
"This is a new concept," says Lee. "The protein itself is the soldier that does the job, but now we're thinking it's not just about the soldier, but also where the soldier is deployed."
The research has been published in PNAS.