Disease-causing pathogens are notoriously dangerous foes that have evolved many tricks to thwart the body's immune system. Among the most terrifying are ones that can sneak into the nervous system, even infecting the brain.
Now, a new study from researchers in the UK, Australia, and Singapore has captured a timelapse of destruction in lab-grown zebrafish, showing how a fungus that causes a rare type of meningitis spreads out of the bloodstream and into the brain.
As if squeezing between the tightly packed cells of the blood-brain barrier or hitching a ride inside immune cells bound for the brain (like many other pathogens do) wasn't enough, it seems one meningitis-causing microbe that goes by the name of Cryptococcus neoformans has another trick in the bag: blocking and bursting small blood vessels in the zebrafish brain.
"The brain has very complex and effective defenses against microbes," says senior author Simon Johnston, an infectious disease researcher at the University of Sheffield, UK.
"But we have identified a simple and effective method that microbes may use to escape the blood and enter the brain."
Meningitis is a life-threatening disease caused by bacterial or fungal infections of the brain and spinal cord that lead to swelling and inflammation of the meninges, the protective membrane cloaking the central nervous system.
Meningitis can be fatal if not recognized and treated quickly; a problem made worse by the growing threat of antimicrobial-resistant infections, which are now the third leading cause of death worldwide.
Organ transplant recipients, folks with HIV/ AIDS, and other people with weakened immune systems are most at risk of contracting fungal meningitis, which doesn't spread directly between people and can only be picked up from the environment.
While there can be many microscopic culprits of meningitis, many of which are found in bug-filled soils, C. neoformans causes fungal meningitis, which is rarer than other forms of the disease that are preventable with vaccination. The pathogen is known to damage blood vessels, but exactly how remained unclear.
"We started this research because we knew there was unexplained blood vessel damage in some meningitis patients," says Johnston, who in past work has exposed how C. neoformans manipulates immune cells.
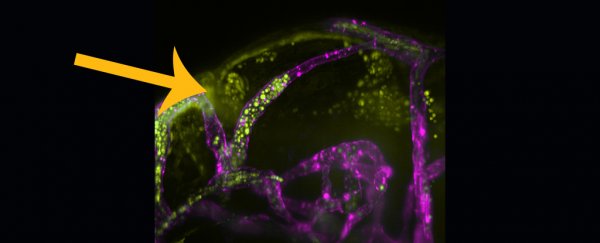
In this study, Johnston and colleagues used fast-growing, see-through zebrafish to watch how C. neoformans behaves in blood vessels, which are stained purple in the image below. The C. neoformans fungal spores are depicted in fluorescent yellow.
Using high-resolution, live-cell imaging techniques, the researchers visualized the fluorescently-labeled blood-borne fungal invaders over several days. Microbes became lodged in blood vessels, restricting blood flow and blocking blood supply. As the infectious microbes multiplied, the blood vessels stretched and ruptured, releasing C. neoformans into the zebrafish brain tissue.
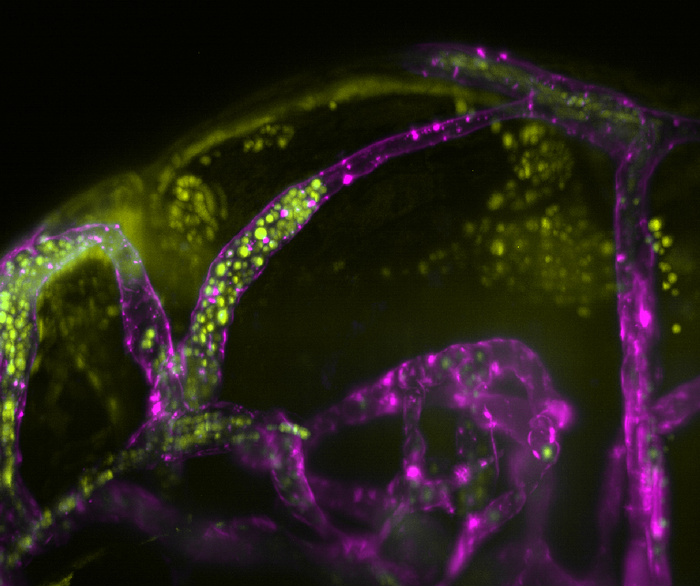 Yellow-tagged C. neoformans bursting out of purple-stained blood vessels. (University of Sheffield)
Yellow-tagged C. neoformans bursting out of purple-stained blood vessels. (University of Sheffield)
Even single C. neoformans cells could wedge in small blood vessels, seeding blood-vessel-blocking fungal masses; these looked eerily similar to fungal or bacterial masses found next to brain capillaries on post-mortem reports of people who had died of the disease.
In the zebrafish, blockages not only increased local blood pressure but as blood flow backed up, the fungal obstruction also increased the tension of the walls of nearby blood vessels, making them more prone to rupture.
"Previous research has focused on how microbes can break down the defenses of the brain or use immune cells as a route into the brain," says Johnston. "We can demonstrate how, for some microbes, damaging blood vessels is a very effective method of invasion."
On top of that – and this is most insightful – the team also found that C. neoformans masses built up on average two days before blood vessels burst, a tight window in which doctors might possibly intervene to stop an infection before the damage is done.
"Infections causing meningitis can be treated with antimicrobials, but patients are often very ill and a lot of damage can be caused before treatment is effective," says Johnston.
Hopefully, understanding how fungal meningitis spreads in the brain will lead to new therapies that help limit damage to the brain's blood vessels while antifungal treatments work to clear the infection.
Any such treatments are just a pipe-dream at this stage, as the imaging experiments were done in teeny tiny zebrafish.
Nevertheless, Johnston and colleagues think their findings might be relevant to other types of infections that damage or rupture blood vessels, although they note these mechanisms probably vary between microbial species.
The study was published in PLOS Pathogens.