We now know how gold crystals start to form at the atomic scale.
For the first time, scientists have observed – and filmed! – the first milliseconds of gold crystal formation and found that it's much more complicated than previous research suggested. Rather than a single, irreversible transition, the atoms come together and fall apart multiple times before stabilizing into a crystal.
This discovery has implications for both materials science and manufacturing, as it bolsters our understanding of how materials come together out of a messy pile of atoms.
"As scientists seek to control matter at smaller length scales to produce new materials and devices, this study helps us understand exactly how some crystals form," explained physicist Peter Ercius of the Lawrence Berkeley National Laboratory.
According to the classic understanding of nucleation – the very first part of crystal formation, in which atoms begin to self-assemble – the process is a pretty linear one. You put a bunch of atoms together under the right conditions, and they'll gradually build themselves into a crystal.
This process, however, is not easy to observe. It's a dynamic process that happens on extremely small scales, both spatially and temporally, and often has an element of randomness involved. But our technology has improved to the point that we can now observe processes on the atomic scale.
Just earlier this year, a team of Japanese scientists revealed that they'd been able to observe salt crystal nucleation. Now a Korean and American team led by engineer Sungho Jeon of the Hanyang University in the Republic of Korea has done the same with gold.
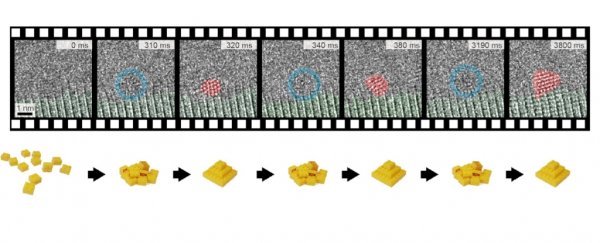
On graphene support films, the team grew tiny nanoribbons of gold cyanide, using one of the world's most powerful electron microscopes to observe it, Berkeley Lab's TEAM I. At speeds of up to 625 frames per second (fps) – extremely fast for electron microscopy – TEAM I captured the first milliseconds of nucleation in incredible detail.

The results were surprising. Gold atoms would come together into a crystal configuration, fall apart, and come together again in a different configuration, repeating the process several times, fluctuating between disordered and crystalline states before stabilizing.
It's not dissimilar to what the Japanese scientists observed with the salt crystals, actually; those atoms, too, fluctuated between featureless and semi-ordered states before coming together into a crystal. But that process was filmed at 25 fps; the gold atoms fluctuated much, much faster.
Only the 625 fps detector speed had a hope of catching it, according to Ercius.
"Slower observations would miss this very fast, reversible process and just see a blur instead of the transitions," he said.
So what causes it? Heat. Nucleation and crystal growth are exothermic processes, which release energy in the form of heat into their surroundings. Think of a really teeny tiny bomb. This repeatedly melts the crystal configurations, which try to reform.
But the reforming process is not helped by the recurrent collisions of incoming atoms that disrupt the cluster of atoms dynamically. Eventually, though, the atoms come together in a way that can withstand the heat released by them doing so.
Et voila! We have a stable gold crystal onto which more atoms can build without collapsing back into the disordered state.
"We found that crystal nucleation of gold clusters on graphene progresses through reversible structural fluctuations between disordered and crystalline states," the researchers wrote in their paper.
"Our findings clarify fundamental mechanisms underlying the nucleation stage of material growth including thin-film deposition, interface-induced precipitation, and nanoparticle formation."
Their next step is to develop an even faster detector in the hope of finding even more hidden nucleation processes.
The team's research has been published in Science.