Here on Earth, when you boil a liquid, it evaporates and turns into a vapour until it's cool enough to condense again. But astronauts on the International Space Station (ISS) have shown that something else entirely is happening in a microgravity environment.
They found that vapour condensed into a liquid, even when the temperature was 160 Kelvin above the substance's normal boiling point - a new phenomenon that's stumped scientists.
Previous research has already shown that microgravity has strange effects on boiling liquids. On Earth, a process called convection plays a crucial role in heat distribution - gravity causes cooler parts of the liquid to sink, while the hotter parts rise, and this spreads the heat around inside the liquid.
But in microgravity environments, such as on board the ISS, that doesn't happen.
To overcome this, the ISS has devices called heat pipes - partial vacuums that transfer heat from somewhere hot to somewhere cooler without the use of a mechanical pump.
They work by containing a small amount of liquid that evaporates off a hot surface, and then condenses back into a liquid when it comes into contact with a cooler surface, thereby redistributing the heat.
Through capillary action, the liquid then travels back to the hot surface to repeat the process indefinitely.
Except this new study by researchers from the Rensselaer Polytechnic Institute in New York and NASA's Glenn Research Centre shows that might not actually be happening on board the ISS.
In fact, the vapour is doing the opposite of what you'd expect - it continues to condense at the heated end, which is an issue for the future of engineering systems.
"Traditional heat pipes are divided into three zones: evaporation at the heated end, condensation at the cooled end, and intermediate or adiabatic in between," the researchers write in Physical Review Letters.
"The microgravity experiments reported herein show that the situation may be dramatically more complicated."
The new study builds on previous observations in 2015, when researchers noticed that increasing the heat input to a heat pipe didn't cause the device to dry up at that end as you'd expect - but instead caused liquid to accumulate there.
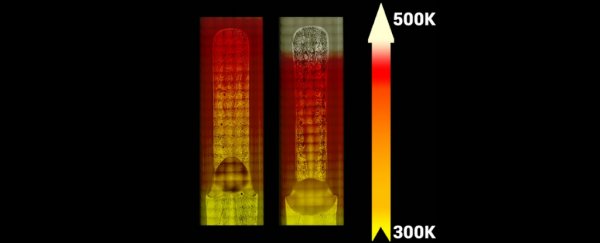
To figure out what was going on, researchers conducted a new experiment on board the ISS using pentane in a heat pipe. They found that as they increased the amount of heat input to the surface, the amount of condensation increased - contrary to what you'd expect.
This continued to happen, even at temperatures of up to 160 K above the normal boiling point for pentane - after that it wasn't safe to continue the experiment.
"The hotter the surface, the more vapour was condensed onto it," the researchers explain.
Liquid above its boiling point is generally said to be in a 'superheated' state, and the researchers describe the hot end of the pipe as being "flooded with superheated liquid" in this experiment.
For now, the researchers aren't entirely sure why this is the case, but their theoretical explanation is that it's something to do with a phenomenon known as the Marangoni effect.
This happens because of the capillary structures built into the heat pipes, and it results in cooler liquid with higher surface tension 'pulling' hotter liquid towards it.
Basically, the hypothesis is that because the surface tension isn't uniform along the heat pipe, the temperature gradient varies over its surface.
"In the end, the effect produces Marangoni-driven flows - one from the heated end to the cooled end, and another from the centre of the pipe to its edges," explains Lisa Zyga over at Phys.org.
"These flows occur even in the hot 'evaporation zone' of the pipe, and they generate an instability in the liquid layer that reinforces the condensation."
Interestingly, this phenomenon does occur on Earth as well, but on a much smaller scale because gravity restricts the return of liquid from the cooled end of a heat pipe to the heated end.
The team now needs to continue investigating exactly what's going on, and test their Marangoni hypothesis in microgravity.
"We, and a number of others, have shown that adding a second chemical component to the system can negate some of the detrimental features observed during operation with a pure fluid," one of the researchers, Joel Plawsky, told Zyga.
"We will be trying experiments, similar to the ones we have already run, with fluid mixtures."
It's not just interesting physics - understanding how this works could be the key to building safer spacecraft cooling systems in future. And we're definitely going to need them.
The research has been published in Physical Review Letters.