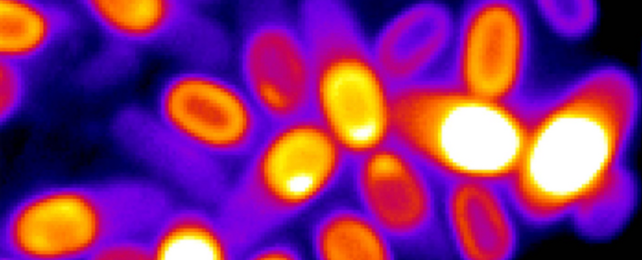
Scientists have detected unexpected activity in dormant bacteria spores, showing for the first time that even when they're physiologically 'dead', the organisms are still aware of their surroundings.
Using a stored supply of charged particles for energy, rather than their usual fuel, the bacteria could actively respond to tiny changes in nutrient levels to determine a prime time to wake.
The discovery challenges our understanding of not just how disease spreads, but also how life could survive in extreme states here on Earth and beyond.
"This work changes the way we think about spores, which were considered to be inert objects," says molecular biologist and lead researcher Gürol Süel from the University of California San Diego.
"We show that cells in a deeply dormant state have the ability to process information. We discovered that spores can release their stored electrochemical potential energy to perform a computation about their environment without the need for metabolic activity."
The implications also spread beyond disease management here on Earth – it's often thought that one way we may encounter extraterrestrial life is in similar dormant states.
"If scientists find life on Mars or Venus, it is likely to be in a dormant state and we now know that a life form that appears to be completely inert may still be capable of thinking about its next steps," says Süel.
Bacteria are generally pretty tough, but one of their key survival tactics is being able to shut down into spores, where they can remain in a dehydrated, dormant state surrounded by a protective coating for hundreds of years – surviving extreme heat, pressure, and even outer space.
This isn't just a deep sleep, either. Essentially, the bacteria are physiologically dead, with no metabolism.
But somehow they know when conditions are right to wake up again.
Spores are how diseases such as anthrax, caused by the Bacillus anthracis bacteria, can survive long periods without water or nutrients in the mail – within moments of exposure to suitable conditions, they're able to rehydrate and restart their metabolism, allowing them to become infectious again.
But how exactly do spores know when to wake up? Responding to every drop of moisture or whiff of nutrient could mean a lot of wasted energy if the good times don't continue for long. Waiting for a feast might also mean lost opportunities, though.
To tease this out further, Süel and his team tested thousands of dormant Bacillus subtilis spores. The bacteria are considered non-harmful to humans and also happen to hold the record for the longest time surviving in space.
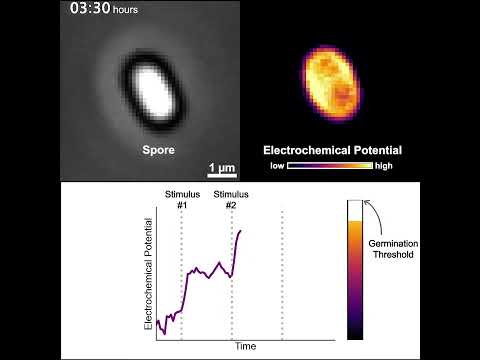
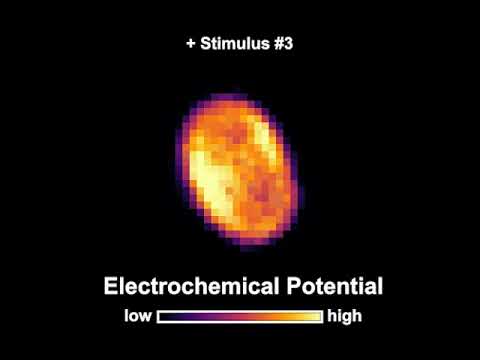
They measured whether the spores could pick up on multiple short-lived pulses of nutrients being sent into their environment – signals that generally wouldn't be strong enough to trigger a spore to come back to life.
As predicted, one or two of these nutrient pulses alone wasn't enough to wake up the bacteria. But over time, a cumulative effect seemed to occur; somehow, the bacteria were somehow able to keep score and switch on again after a certain number of signals.
The team was also able to monitor changes in the activity of the spores in response to these short-lived signals, and showed that the bacteria were using stored energy in the form of potassium ions (K+) with each input.
This is sort of like a capacitor in a circuit, storing the energy for later use.
Using a mathematical model to explain what was happening, the team showed that each signal triggered the release of potassium ions, and over time the potassium ions became strong enough that it kickstarted the reawakening of the bacteria. They called this a 'integrate-and-fire' activation model.
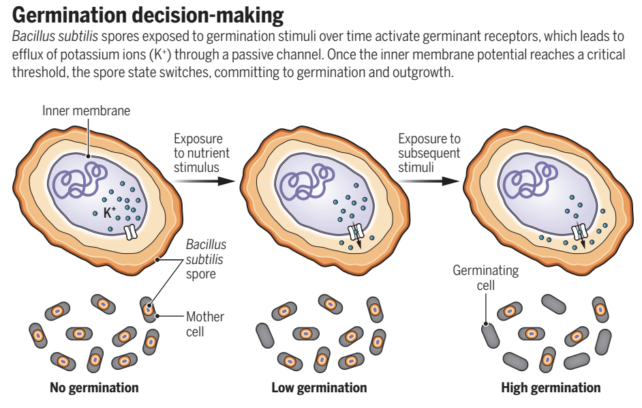
This is known as a cumulative signal processing strategy, and it stops the bacteria from waking up too soon if conditions aren't quite right.
"These findings reveal a decision-making mechanism that operates in physiologically inactive cells," the researchers write in their paper.
In some ways, this is a pretty familiar evolutionary strategy.
"The way spores process information is similar to how neurons operate in our brain," says Süel.
"In both bacteria and neurons, small and short inputs are added up over time to determine if a threshold is reached. Upon reaching the threshold spores initiate their return to life, while neurons fire an action potential to communicate with other neurons."
However, unlike neurons, which are incredibly energy-hungry cells, the spores were able to do this without any metabolic energy at all, only the stored potassium.
In an accompanying Perspectives piece, microbiologists Jonathan Lombardino and Briana Burton from the University of Wisconsin-Madison say that further study across other organisms known to enter a similar spore-like state, such as fungi, could help further tease out what this means for life more broadly.
"For example, could the extreme longevity of so-called superdormant spores be traced to their initial K+ concentrations?" they write.
There's more work to be done, but the discovery challenges our understanding of dormant bacteria as being inert – and also reframes how we may evaluate signs of extraterrestrial life in the future.
The research has been published in Science.