Scientists have singled out which protein forms the characteristic clumps of a type of early-onset dementia where no firm suspect was known before.
This discovery reportedly transforms understanding of the molecular basis of frontotemporal dementia (FTD), the second-most common form of dementia after Alzheimer's, with symptoms typically emerging earlier: in a person's late 40s or 50s.
People diagnosed with FTD experience behavior, personality, language, and movement changes caused by the slow degeneration of the frontal and temporal lobes of the brain. Memory problems can come later as the disease spreads into other brain regions.
But without knowing the true makeup of the tangled protein deposits seen in some of the rarer cases of FTD, researchers had little in the way of targets to explore for potential therapies.
"It is a rare finding of a new member of the small group of proteins known to aggregate in neurodegenerative disease," says Benjamin Ryskeldi-Falcon, a molecular biologist at the MRC Laboratory of Molecular Biology (LMB) in the UK, who led the study.
Clumping proteins are what unites neurodegenerative diseases, and what distinguishes them.
Like amyloid-beta and tau proteins get tangled in Alzheimer's disease, alpha-synuclein in Parkinson's, SOD1 protein in amyotrophic lateral sclerosis (ALS), and Huntington, the eponymous protein that gives Huntington's disease its name, form sticky globs of different shapes in diseased brain tissue.
Different subtypes of FTD, which display different symptoms, are also defined by knotty clumps of TDP-43 protein and tau fibrils, respectively.
But in around 10 percent of FTD cases, no such protein has been identified. The clumps were visible, but no one knew what they were made of.
Researchers suspected one protein called FUS because of the overlap between FTD and ALS, but no genetic mutations in FUS had been found in cases of FTD to suggest it was the misfolded protein to blame.
To get a better look, Ryskeldi-Falcon, Stephan Tetter, a protein scientist at MRC-LMB, and their colleagues extracted protein samples from brain tissue of four patients who had died of FTD and donated their brains to research.
When Tetter imaged the protein samples using cryo-electron microscopy – a technique that bombards individual flash-frozen proteins with electrons to reveal their form – a single unique structure emerged.
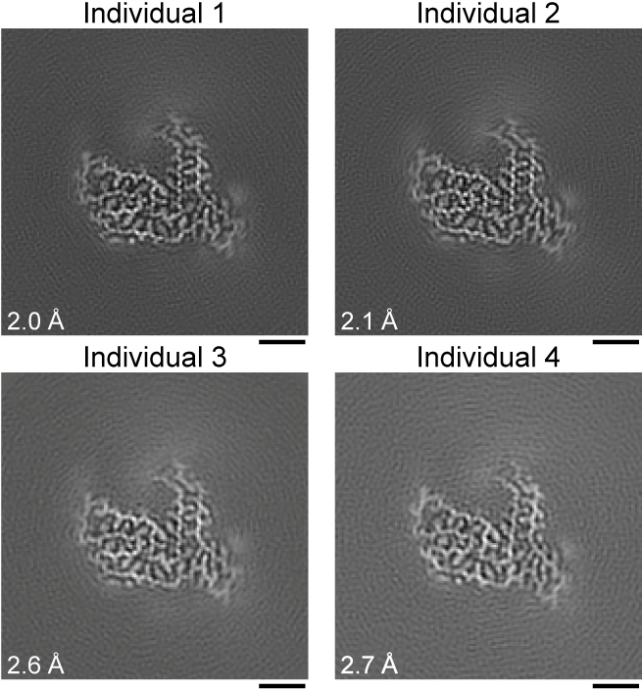
It wasn't until they sequenced those proteins, stringing together the building blocks that give any protein its shape, that Tetter and colleagues realized the protein wasn't FUS; it was another protein from the same protein family called TAF15.
"This is an unexpected result because, before this study, TAF15 was not known to form amyloid filaments in neurodegenerative diseases and no structures of the protein existed," says Tetter.
While there's lots more to understand about TAF15, and the results of this study need to be validated in more patient samples, it's welcome news for the 10 percent of people with this unusual subtype of FTD.
The finding sets scientists off on a path of discovery that started almost 40 years ago for Alzheimer's disease, and about a quarter of a century ago for Parkinson's disease, when researchers untangled which proteins formed the toxic clumps in each disease.
That path is a long and bumpy road, though, littered with setbacks and snags.
In the case of Alzheimer's disease, a string of disappointing clinical trials peppered by some exciting but uncertain progress, and an explosive investigation calling into question the reigning theory of what causes the disease, have brought the field to a critical juncture.
It's early days yet for TAF15 and FTD, and we'll have to see how the research unfolds.
The study has been published in Nature.